Double-target DNA vaccine and constructing method thereof
A DNA vaccine and dual-targeting technology, applied in recombinant DNA technology, DNA/RNA fragments, pharmaceutical formulations, etc., can solve the problem that scholars only focus on research fields, research strategies, low efficiency of gene introduction, and failure to learn from different fields Research strategy and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, preparation and identification of Escherichia coli bacterial ghost (BG)
[0047] 1. Construction of expression plasmids for heat-induced expression of phage E protein
[0048] The E protein gene of PhiX174 bacteriophage is connected into pHH43 ( H K, et al. Therecombinant Azotobacter vinelandii mannuronan C-5-epimerase AlgE4 epimerizes alginate by a nonrandom attack mechanism. JBiol Chem 1999; 274: 12316-12322), the specific method is as follows:
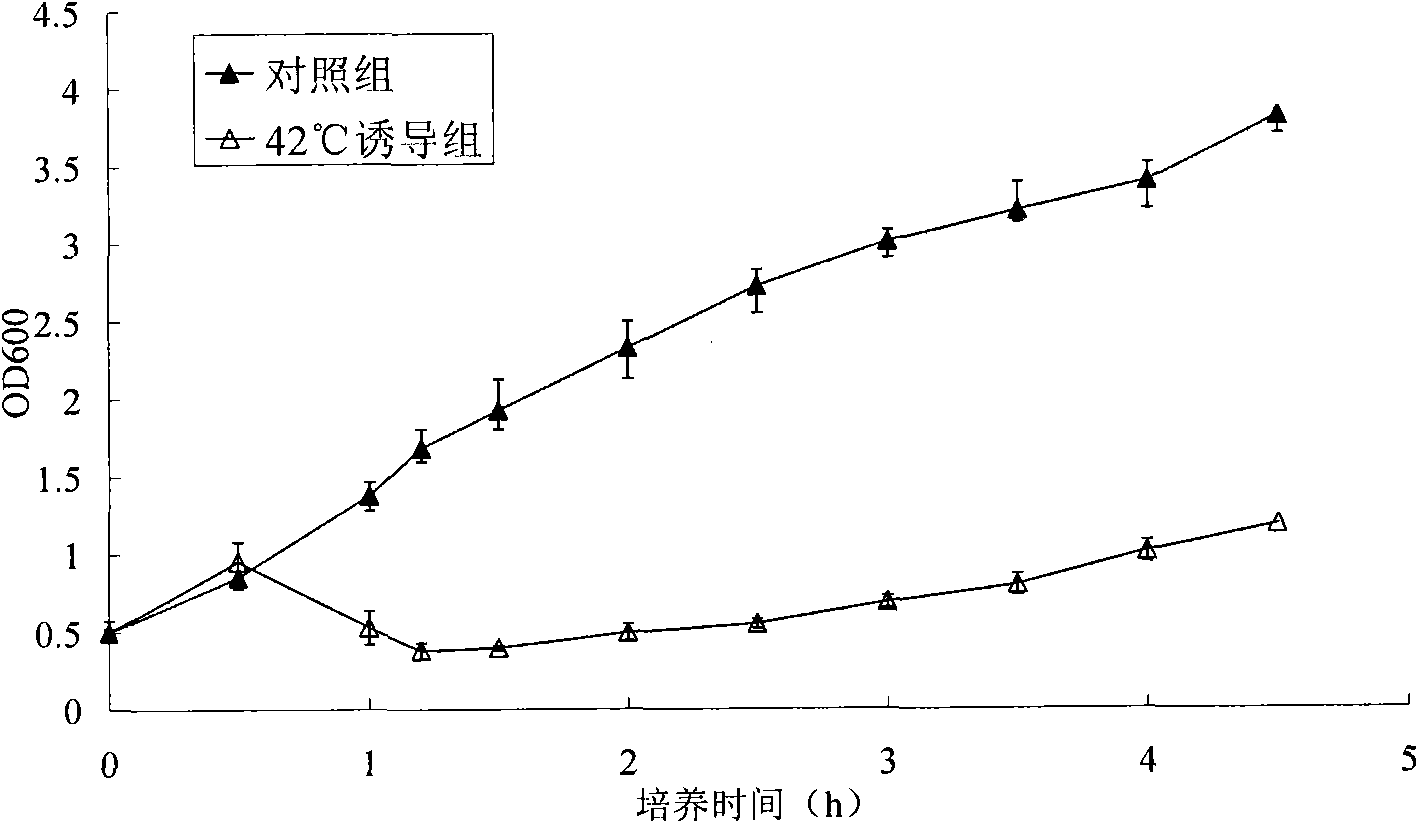
[0049] After the cleavage gene box (E-box) (including temperature-sensitive transcriptional regulatory region (cI857), lambda promoter region, E gene region) was connected in series, kpn I and EcoR V restriction sites were added upstream and downstream, respectively, and then and The expression vector pHH43 was digested and ligated, and the identification and sequencing were correct. For the sequence of the E gene cleavage cassette, see figure 2 . Specific method: Carry out double digestion of the E-box fra...
Embodiment 2
[0059] Embodiment 2, construct the eukaryotic expression vector of mouse invariant chain (mIi)
[0060] Plasmid pQE31-mIi (Bischof F, et al.Melms A.Specific treatment of autoimmunity with recombinant invariant chains in which CLIP is replaced by self-epitopes.Proc Natl Acad Sci US A.2001; 98(21):12168-73) as Template, using the PCR method to obtain the mIi coding gene (when designing the PCR primer sequence, introduce Xho I and EcoR I restriction sites at the 5' ends of the upstream and downstream primers respectively, and the specific sequence is
[0061] P1: 5'-GCG CTCGAG ATGACGGATCCGCATGCGAGCT-3' and
[0062] P2: 5'-CGC GAATTC GCAGGGTGACTTGACCCAG-3').
[0063] The method is: in a 50 μl reaction system, primers (1 μl each for forward and reverse primers), template (1 μl), high-fidelity DNA polymerase (0.25 μl), dNTP (2.5 mM, 4 μl), 10× amplification Adding buffer (5μl) and deionized water (36.75μl) were shaken and mixed, after centrifugation, pre-denaturation at 94°C f...
Embodiment 3
[0065] Embodiment 3, construct the FMDV-VP1 vector of endogenous targeting
[0066] 1. Select and synthesize the dominant epitope of foot-and-mouth disease virus (FMDV)
[0067] FMDV is a single-stranded positive-strand RNA virus, and its structural protein VP1 has multiple B cell and T cell epitopes, which can induce a strong immune response; among them, VP1 proteins 21-40, 141-160, 200-213 The antigenic epitope has the strongest antigenicity, and all kinds of serotype FMDV have this epitope, so it is a very important DNA vaccine candidate sequence. It is planned to use the 21-40+141-160 and 141-160+200-213 sequences as targets to construct corresponding expression vectors.
[0068] 2. Build the carrier
[0069] Such as Figure 8 As shown, use pDSRed-mIi as the eukaryotic expression vector (constructed in Example 2), use the 21-40+141-160, 141-160+200-213 sequences to replace the CLIP region of mIi, and construct a eukaryotic expression vector for VP1 Expression vector. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com