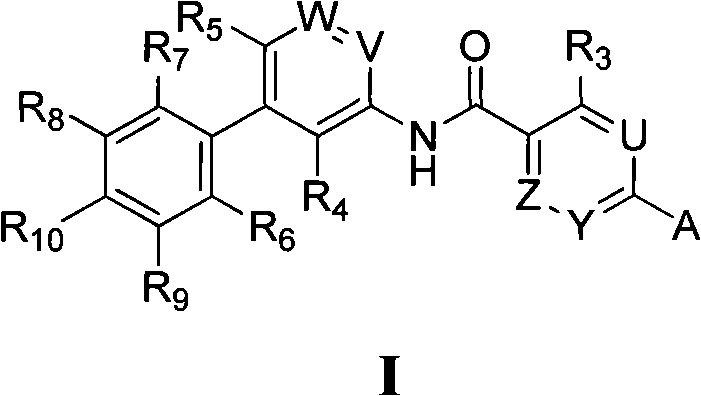
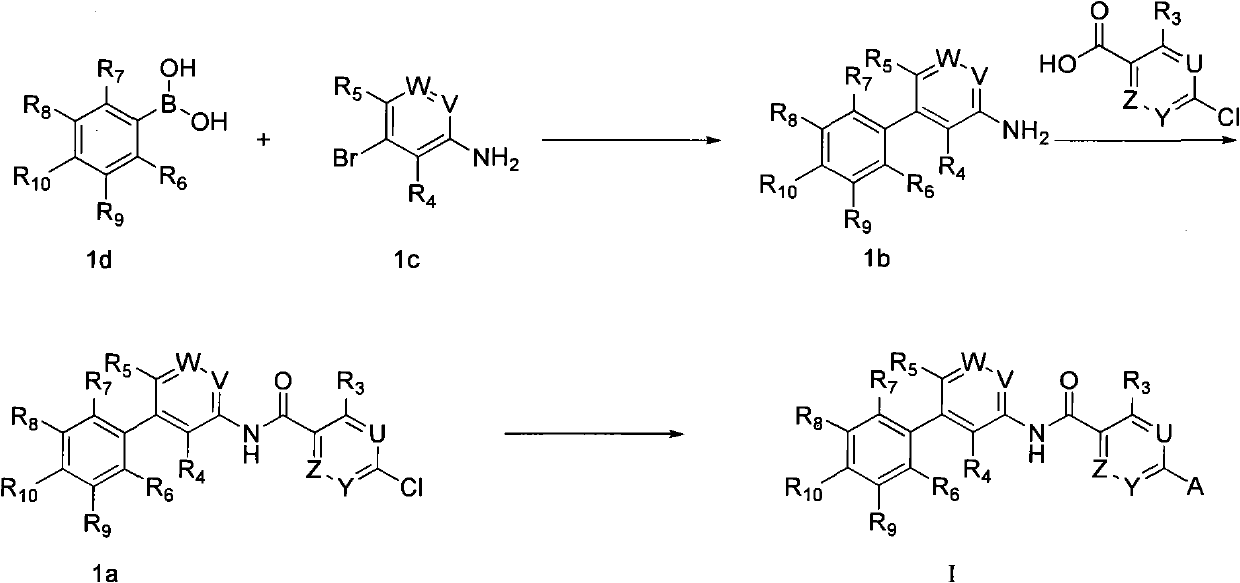
Compound as Hedgehog path inhibitor, medicine composition containing same and application thereof
A technology for compounds and solvates, applied in the field of compounds and compositions containing the compounds, can solve problems such as insignificant therapeutic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0168] The experiments, synthesis methods and intermediates described below are illustrative of the present invention and do not limit the scope of the present invention.
[0169] The starting materials used in the experiments in the present invention were either purchased from reagent suppliers or prepared from known materials by standard methods. Unless otherwise stated, the examples herein apply the following conditions:
[0170] 1) The unit of temperature is Celsius (°C); the definition of room temperature is 18-25°C;
[0171] 2) Dry the organic solvent with anhydrous magnesium sulfate or anhydrous sodium sulfate; use a rotary evaporator to spin dry under reduced pressure and elevated temperature (for example: 15mmHg, 30°C);
[0172] 3) Silica gel is used as a carrier during column chromatography separation, and TLC indicates a silica gel thin-layer plate;
[0173] 4) Usually, the progress of the reaction is monitored by TLC or LC-MS;
[0174] 5) Identification of the f...
preparation Embodiment 1
[0175] Preparation Example 1: N-{2-Methyl-3-[4'-(trifluoromethyl)phenyl]}phenyl-6-morpholine nicotinamide Synthesis
[0176]
[0177] 1) 3-amino-2-methyl-4'-trifluoromethylbiphenyl of preparation formula 3:
[0178] Weigh 3-bromo-2-methylaniline (1.0g, 5.4mmol, compound of formula 1), 4-trifluoromethylphenylboronic acid (1.3g, 6.8mmol, compound of formula 2), bistriphenylphosphine dichloride Palladium chloride (0.4 g, 0.54 mmol) and sodium carbonate (1.7 g, 16.0 mmol) were placed in a microwave synthesis reaction tube under nitrogen protection, and heated to 120° C. for 30 min by microwave. After the reaction was completed, the insoluble matter was removed by filtration, the filtrate was diluted with water, extracted with ethyl acetate, the organic phase was washed with brine, and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure, and the crude product was purified by column chromatography (n-hexane:ethyl acetate 8:1) to obtain a yellow...
preparation Embodiment 2
[0189] Preparation Example 2: N-{2-methyl-3-[4'-(trifluoromethoxy)phenyl]}phenyl-6-[(2R,6S)-2,6-dimethylmorph Synthesis of Phylo]pyridazine-3-amides
[0190]
[0191] 1) N-(3-bromo-2-methylphenyl)-6-chloropyridazine-3-amide of formula 3:
[0192]Under ice bath, 3-bromo-2-methylaniline (372.1 mg, 2.0 mmol, compound of formula 1), 6-chloropyridazine-3-acid (317.1 mg, 2.0 mmol, compound of formula 2), 1-( 3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI, 766.8 mg, 4.0 mmol) was mixed and dissolved in 10 mL of pyridine, stirred at room temperature overnight, pyridine was removed under reduced pressure, and the residue was washed with ethyl acetate Dissolved, the organic phase was washed with water (3×5 mL), and dried over anhydrous sodium sulfate. It was filtered, concentrated, and purified by silica gel column chromatography (n-hexane:ethyl acetate 5:1) to obtain a white solid (265.0 mg, 40.6%, compound of formula 3).
[0193] 1 H-NMR (300MHz, CDCl 3 )δ: 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com