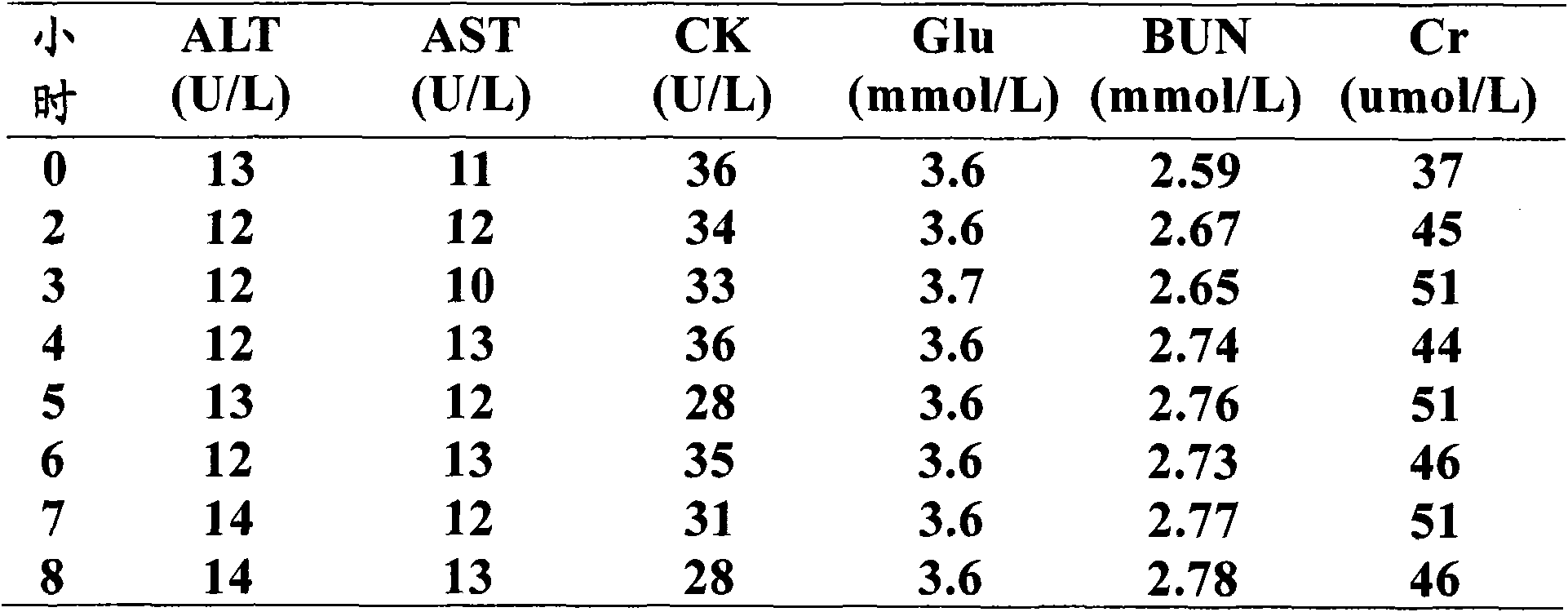
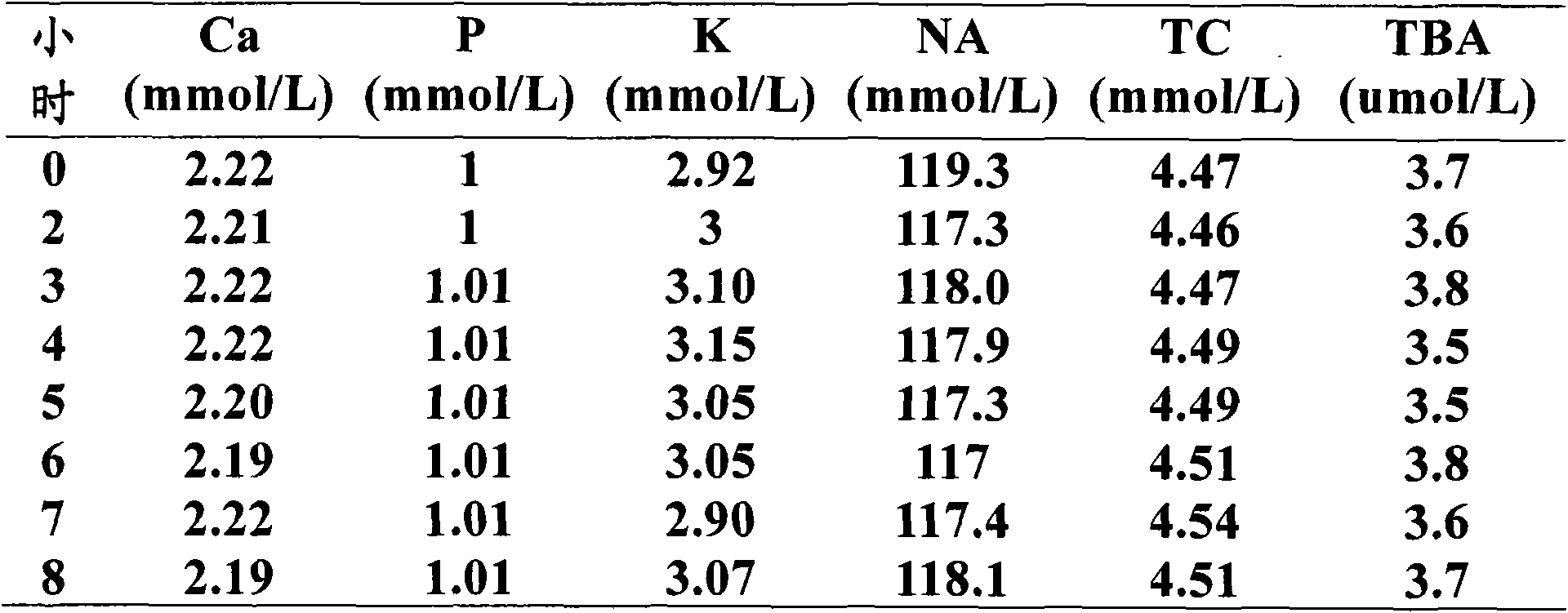
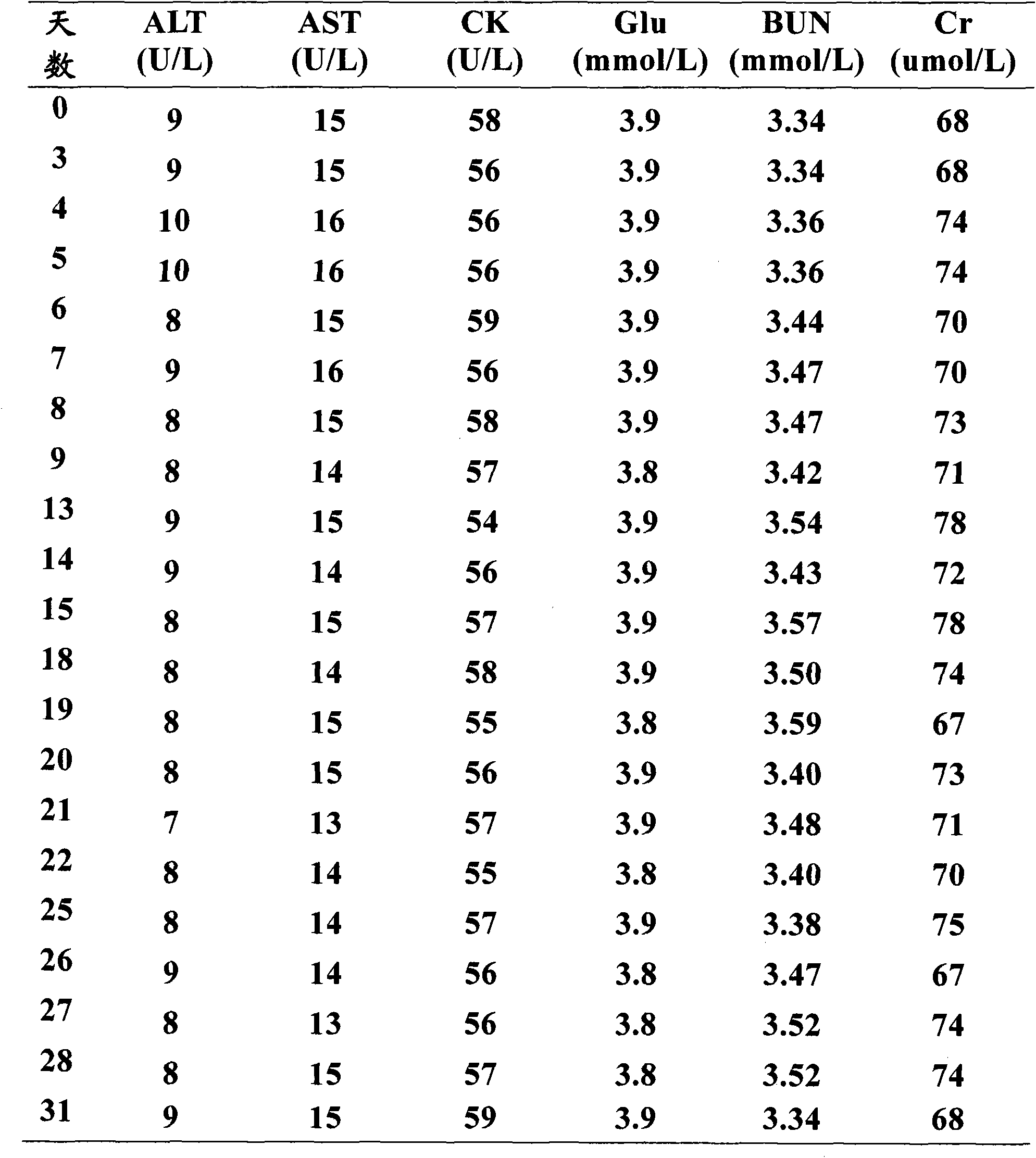
Liquid quality control serum for clinical chemical detection
A technology for quality control of serum and liquid, which is applied in the field of preparing the liquid quality control serum, and can solve problems such as no practical use, unsatisfactory stability, and influence on the development of indoor quality control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0110] Example 1. Preparation of liquid quality control serum
[0111] a) Take 1000ml of serum raw material. The serum raw material comes from healthy volunteers. Its ALT index is normal (below 40U / L), and the following indexes are negative: HBsAg, HBsAb, HBeAg, HBeAb, HBcAb, HCV-Ab, HIV- Ab, and RPR, these serums are all obtained by centrifugation and blood collection using a vacuum blood collection tube with separating gel. Mix these serums thoroughly, store this mixed serum in a refrigerator at -20℃, and store the mixture at -20℃ before use. After the serum is thawed in a refrigerator at 2~8℃ overnight, mix well at room temperature and set aside;
[0112] b) Pretreat the dialysis bag according to the conventional method. Put a dialysis bag with a width of 2 to 3 cm into a beaker, soak it in deionized water for about half an hour, then place the beaker on the induction cooker and boil for 10 minutes, and wash it with deionized water 3 times , Boil for another 10 minutes, wash 3 ...
Embodiment 2
[0118] Example 2. Preparation of liquid quality control serum
[0119] Refer to the method of Example 1 to prepare the liquid quality control serum of this example, but differently, the following parameters are used in the following operations:
[0120] 1) In step d), about 30% of the water content of the serum raw material is removed;
[0121] 2) In step e), a mixture of ethylene glycol and propylene glycol having a dehydration amount equivalent to that in step d) is used, wherein ethylene glycol: propylene glycol = 1:1.5 (v / v).
[0122] The liquid quality control serum thus prepared was stored in a refrigerator at -20°C.
Embodiment 3
[0123] Example 3. Preparation of liquid quality control serum
[0124] Refer to the method of Example 1 to prepare the liquid quality control serum of this example, but differently, the following parameters are used in the following operations:
[0125] 1) In step d), about 35% of the water content of the serum raw material is removed;
[0126] 2) In step e), a mixture of ethylene glycol and propylene glycol having the same amount of dehydration as in step d) is used, wherein ethylene glycol: propylene glycol=1:1.8 (v / v).
[0127] The liquid quality control serum thus prepared was stored in a refrigerator at -20°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com