Electroplating method and apparatus
A plating and electrodeposition technology, used in current conducting devices, electrolytic components, electrolytic processes, etc., can solve the problems of not providing reproducibility of parts, not allowing one-time plating, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
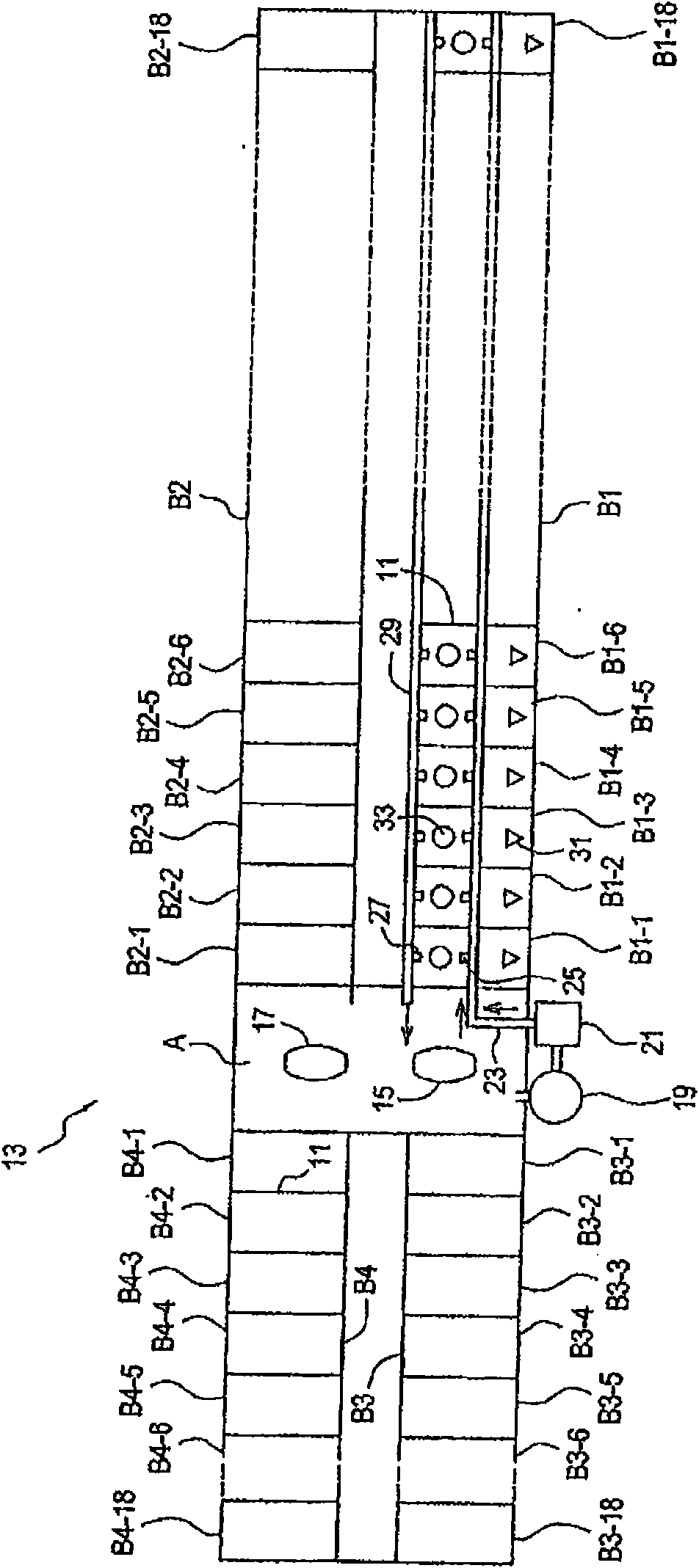
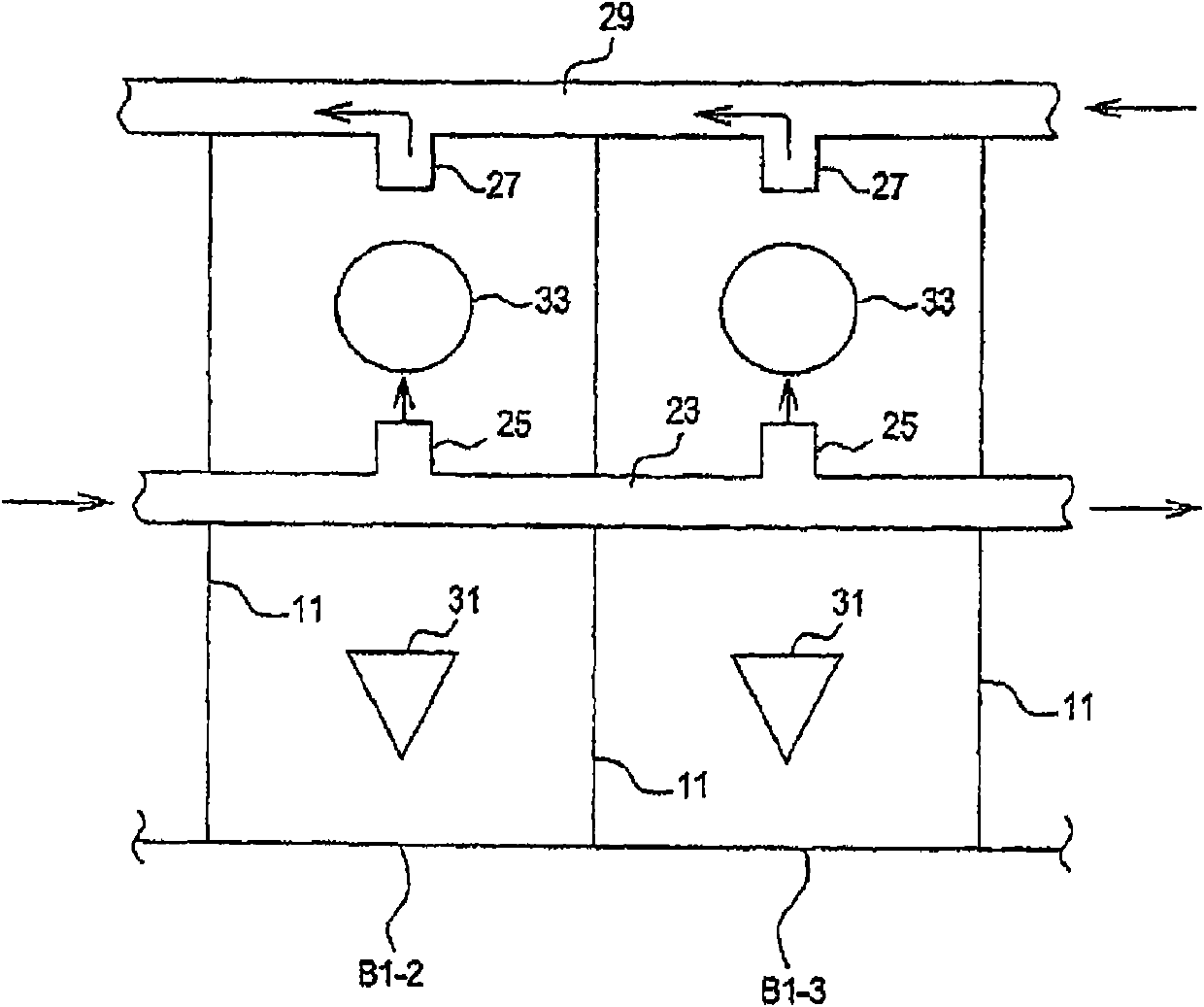
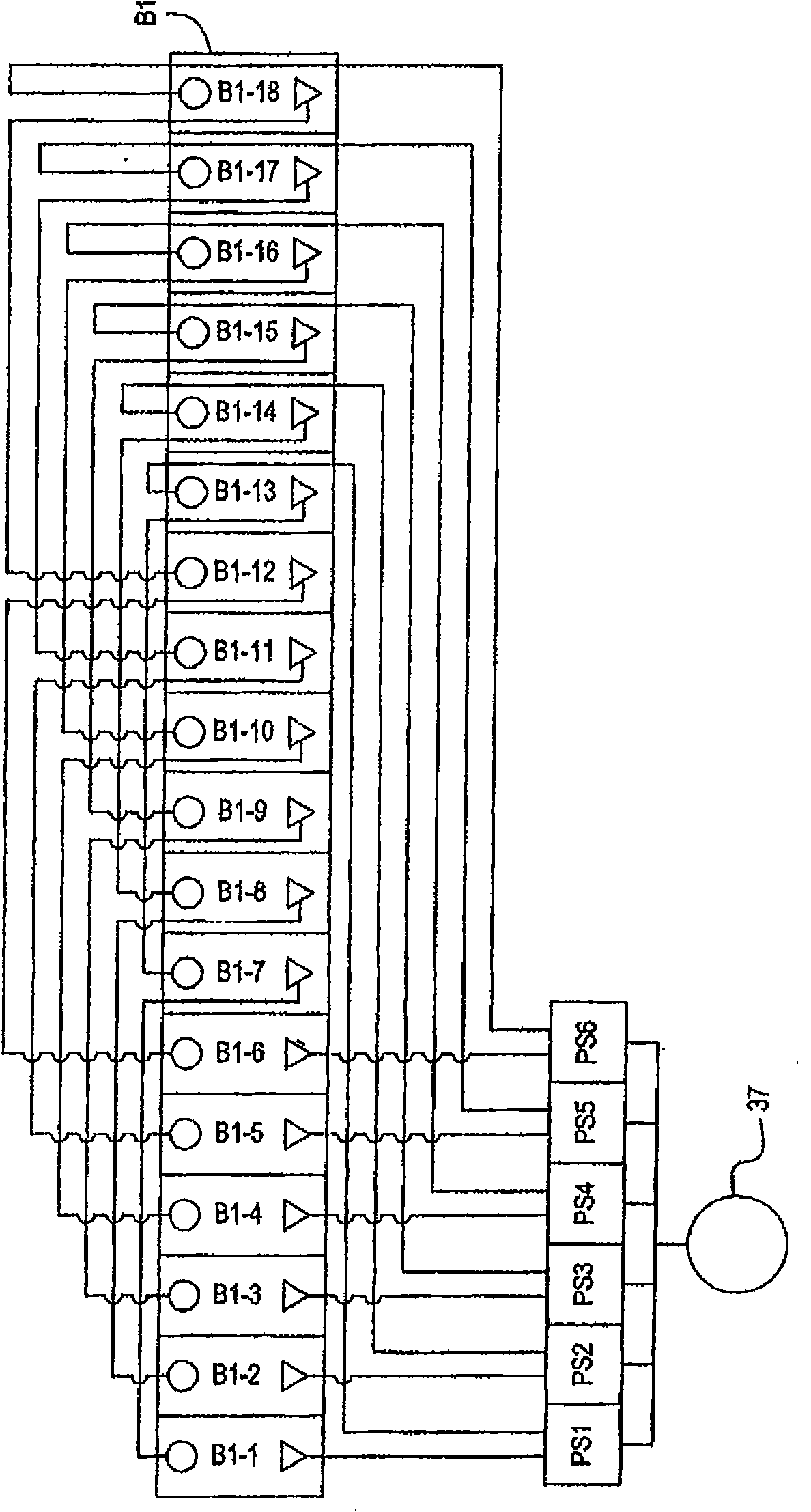
[0196] Parallel plating cells of multiple components in a plating cell system using a common electrolyte
[0197] To illustrate the prior art technique known in the art as rack plating to simultaneously plate parts by electrically connecting all the parts in parallel and controlling the total current supplied to the rack, two different parts were chosen (a celluloid sphere and a flat polyamide pulley). tensile test piece).
[0198] In Experiment 1, table tennis balls (40 mm diameter) made of celluloid were suitably metallized with a Ni film (electroless nickel plating, MacDermid Inc., Denver, Colorado, USA), and thereafter used for the Permalloy gold The modified Watts nickel bath of the use of grain refiners, brighteners, specifically and (Integran Technologies Inc., Toronto, Canada) electroplated a layer of nanocrystalline nickel-iron alloy (n-Ni-20Fe) to an average thickness of approximately 185 microns in 4.5 hours. Soluble Ni spheres (Inco Ltd., Sudbury, Ontario,...
Embodiment 2
[0224] Graphite / epoxy at different coat weights in single and multi-plating cell systems DC polarization curve of lipid duct
[0225] The apparatus used was as described in Background Example 1. In this experiment, the plated part was a metallized graphite / epoxy tube. Figure 4 The variation of the polarization curve of graphite / epoxy tubes is shown with increasing Ni coating weight. The tube was spun at 15 RPM throughout the experiment. Curve 1 shows the DC polarization curve of a graphite / epoxy tube corrected for cell voltage for IR loss of "trans-wall contact". All remaining curves were recorded using through-wall and surface electrical contacts and using shielding. Curve 4 shows the current / voltage response using DC as described and using both through-wall and surface contacts and using shielded / thieving graphite / epoxy tubes before any significant Ni deposition occurs on the outer surface. Curve 3 shows the drop in cell voltage as the Ni coat weight was increased t...
Embodiment 3
[0227] Graphite / epoxy at different coat weights in single and multi-plating cell systems Pulse current polarization curve of lipid duct
[0228] The apparatus used and experiments performed were as described in Background Example 2, except that DC plating was replaced by pulsed current deposition (50% duty cycle). Figure 5 Shown is the change in the polarization curve of the metallized graphite / epoxy tube with increasing Ni coating weight. Curve 1 shows the average plating current for graphite / epoxy tubes with cell voltage corrected for IR loss. Curve 4 shows graphite with 50% duty cycle (8 milliseconds on followed by 8 milliseconds off time) as described and using both through-wall and surface contact and shielding / thieving before any significant Ni deposition occurs on the outer surface / Average current / voltage response of the epoxy tube. Curve 3 shows the decreased cell voltage under the same conditions after the Ni coating weight was increased to 4 grams and curve 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequency | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com