Preparation method of nano-flaky FePO4.2H2O
A technology of fepo4·2h2o and nano flakes, applied in chemical instruments and methods, inorganic chemistry, non-metallic elements, etc., can solve the problem of inability to control the size and shape of lithium iron phosphate, and the inability to effectively control the size and shape of products , It is not conducive to improving the electrochemical performance of lithium iron phosphate cathode materials, etc., to achieve the effect of improving electrochemical performance, simple synthesis method, and good electrochemical performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
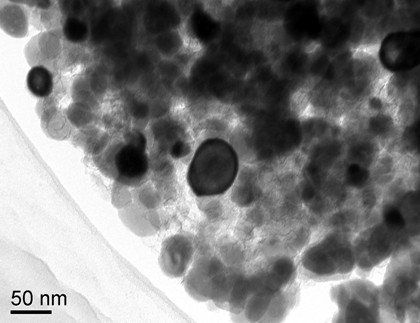
[0029] Take 1.4 g of cetyltrimethylammonium bromide (CTAB) and dissolve it in 20 mL of deionized water to obtain a clear and transparent solution, add 3.0 g of FeCl to the solution 3 ·6H 2 O and stir to dissolve it completely, then add 4mL H 3 PO 4 The solution (85 wt%) was dropped into the above solution and stirred for 1 hour. Afterwards, the transparent solution was heated in a water bath at 90 °C for 24 h. The white precipitate obtained by filtration was washed successively with deionized water and ethanol, and then dried at 100°C for 4 hours. The product obtained by the above synthesis method was characterized by XRD, SEM and TEM, and it was proved that the product was FePO with a monoclinic crystal system and a thickness of 100 nanometers. 4 2H 2 O crystal flakes (the resulting FePO 4 2H 2 The SEM and TEM images of the O crystal flakes are shown in the appendix figure 1 And attached figure 2 ).
Embodiment 2
[0031] Get 1.4 g of cetyltrimethylammonium bromide (CTAB) and dissolve it in 200 mL of deionized water to obtain a clear and transparent solution (the concentration of CTAB is one-tenth of that in Example 1), and add 3.0 g of FeCl to the solution 3 ·6H 2 O and stir to dissolve it completely, then add 4mL H 3 PO 4 The solution (85 wt%) was dropped into the above solution and stirred for 1 h. Afterwards, the transparent solution was heated in a water bath at 90 °C for 24 h. The obtained white precipitate was filtered, washed successively with deionized water and ethanol, and then dried at 100°C for 4 hours. The obtained product was characterized by XRD, which showed that the obtained product was monoclinic FePO 4 2H 2 O crystals. The obtained product was characterized by SEM, which indicated that the obtained product was a flake with a thickness of 30 nm. With the FePO 4 2H 2 O crystal flakes as Fe and P sources supplemented with LiOH·H 2 O is used as the lithium sourc...
Embodiment 3
[0033] Take 1.4 g of cetyltrimethylammonium bromide (CTAB) and dissolve it in 20 mL of deionized water to obtain a clear and transparent solution, add 3.0 g of FeCl to the solution 3 ·6H 2 O and stir to dissolve it completely, then add 1mL H 3 PO 4 solution (85 wt%) was dropped into the above solution (H 3 PO 4The concentration is a quarter of that of Example 1) and stirred for 1 hour. Afterwards, the transparent solution was heated in a water bath at 90 oC for 20 h. The white precipitate obtained by filtration was washed successively with deionized water and ethanol, and then dried at 100°C for 4 hours. The product obtained by the above synthesis method was characterized by XRD and SEM, and it was proved to be FePO with a monoclinic crystal system and a thickness of 50 nanometers. 4 2H 2 O crystal flakes. In this synthetic method, H 3 PO 4 A wide concentration range is possible.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com