Preparation method of di(dibenzalacetone)palladium(0)
A technology of dibenzylideneacetone and acetone is applied in the field of preparation of bis(dibenzylideneacetone)palladium(0), and can solve the problems of long reaction time, difficult operation, and not strictly controlling the amount of ligand added.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
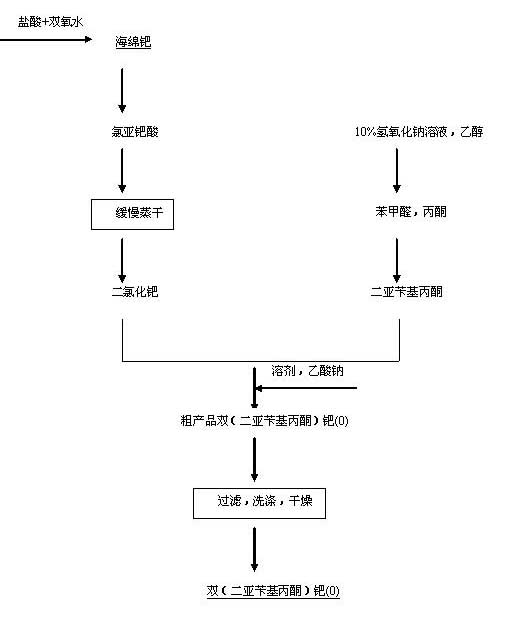
[0015] A kind of preparation method of two (dibenzylideneacetone) palladium (0), the step of preparation process comprises:
[0016] (i) Preparation of palladium dichloride: take a certain amount of palladium sponge, mix and dissolve with hydrochloric acid of 32% concentration and hydrogen peroxide of 30% concentration, lower the temperature of the electric heating plate, and slowly evaporate the obtained chloropalladium acid solution by dissolving Dry to get palladium dichloride;
[0017] (ii) Synthesis of dibenzylideneacetone: Measure a certain volume of 95% ethanol, 100g / L sodium hydroxide solution, and deionized water in a 50L circular glass reactor, and add a certain amount of benzaldehyde in turn under stirring , acetone, control the reaction temperature at 20-40°C, and continue the reaction for 2 hours to form a light yellow precipitate;
[0018] (Ⅲ) the suspension in step (ii) is cooled to room temperature, and is vacuum filtered to obtain the thick product of dibenzy...
Embodiment 1
[0022] Preparation of dibenzylidene acetone: Weigh 600g of sodium hydroxide, dissolve it with 6L of deionized water and add it to a 50L round glass reactor, then add 12L of deionized water, 12L of 95% ethanol, 1.59L of benzaldehyde, and 540ml of acetone ; Stir mechanically, react at room temperature for 2h; filter with suction, recrystallize from absolute ethanol, dry and weigh to obtain 1421.5g of product, yield 81%;
[0023] Product preparation: take 120g palladium sponge, mix and dissolve with 32% hydrochloric acid and 30% hydrogen peroxide, slowly evaporate the resulting solution to dryness to obtain palladium dichloride; mix palladium dichloride, 884.62g dibenzyl Diethyl acetone (prepared for use in advance), 750.00g of anhydrous sodium acetate, and 24L of anhydrous ethanol were added to the jacketed reactor, and the oil bath was heated to 65°C. Continue to react at 45°C for 2 hours, cool to room temperature, filter with suction, wash with absolute ethanol, deionized wate...
Embodiment 2
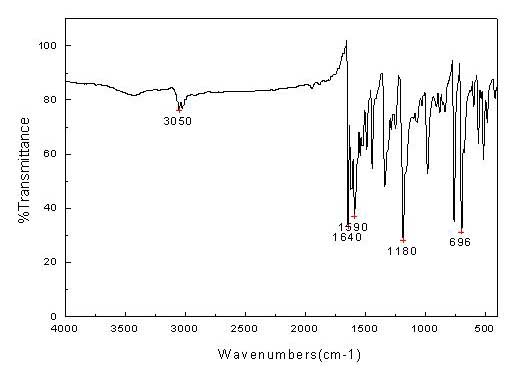
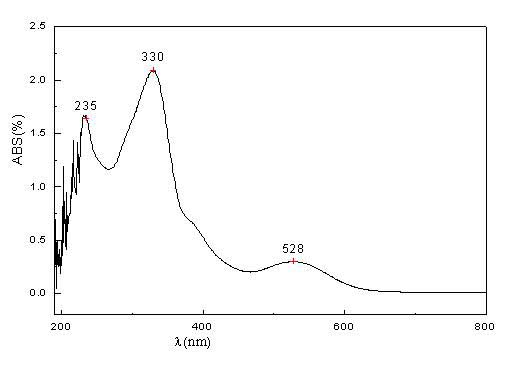
[0025] The specific implementation rules are the same as in Example 1, except that the sponge palladium is 240g, the dibenzylidene acetone is 1769.24g, anhydrous sodium acetate 1500.00g, and absolute ethanol 30L; the oil bath is reacted at 65°C for 30min, and at 45°C for 2h; The rate is 90.9%; the elemental analysis results are C: 70.65%, H: 4.87%, Pd: 18.21%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com