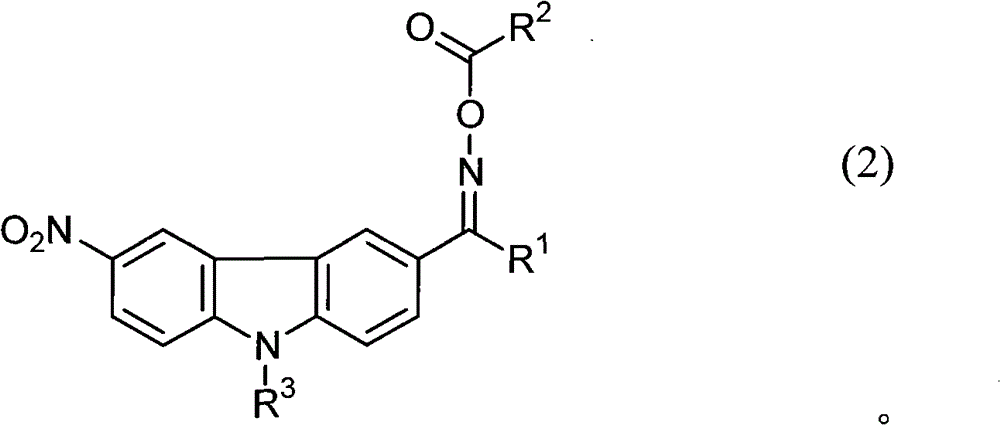
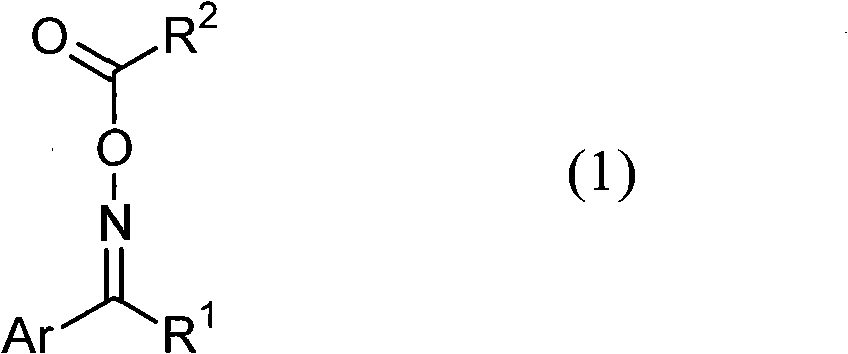Photopolymerization initiator and photosensitive composition
A technology of photopolymerization initiator and photosensitive composition, applied in optics, optomechanical equipment, instruments, etc., can solve the problems of contaminating the calciner, damaging the reliability of color filters, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
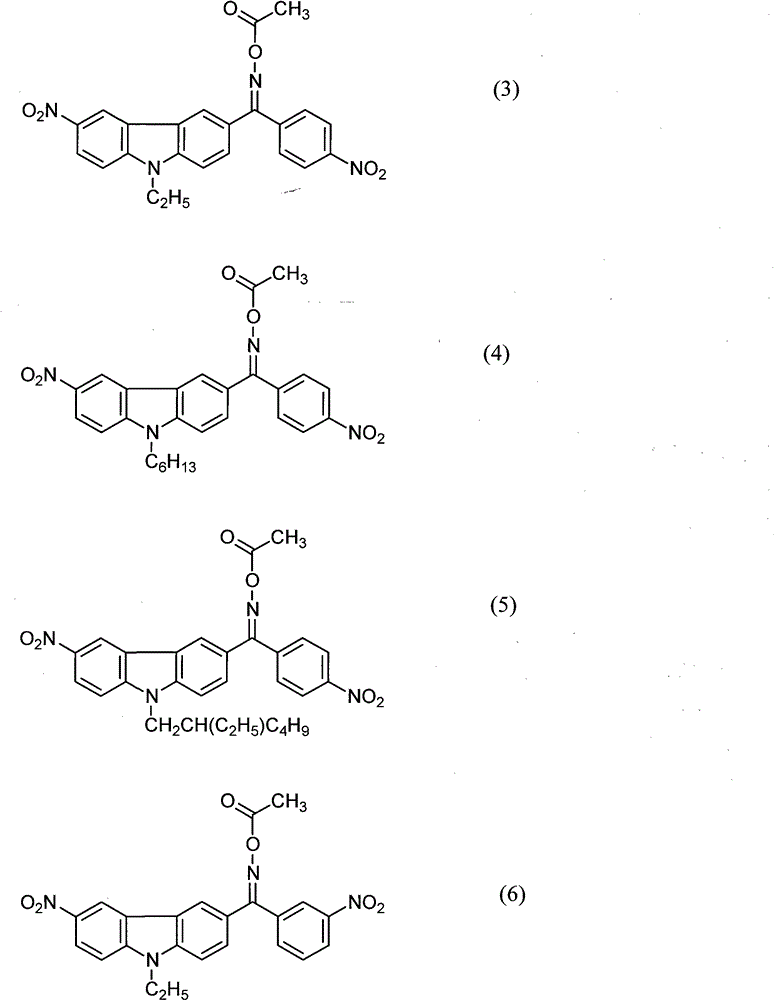
[0044] (9-ethyl-6-nitrocarbazol-3-yl)(4-nitrophenyl)methanone=O-acetyl oxime (photopolymerization initiator of formula (3))
[0045] Synthesis of photopolymerization initiator
[0046] The above-mentioned photopolymerization initiator is synthesized through the reaction of 4 steps below. The reaction was carried out under normal pressure using a glass reactor.
[0047] Step 1: Using nitromethane as a solvent, make 0.10 mol of 9-ethylcarbazole (manufactured by Tokyo Chemical Industry Co., Ltd.), 0.15 mol of 4-nitrobenzoyl chloride (manufactured by Tokyo Chemical Industry Co., Ltd.), and 0.15 mol of aluminum chloride in React at 0 to 5°C for 6 hours. The reaction solution was dropped into ice water, and the precipitated solid was collected and washed thoroughly to obtain a crude product corresponding to 0.07 mol of (9-ethylcarbazol-3-yl)(4-nitrophenyl)methanone .
[0048] Step 2: Using acetic acid as a solvent, react the whole amount of the product in Step 1 and 0.14 moles o...
Embodiment 2
[0057] (9-hexyl-6-nitrocarbazol-3-yl)(4-nitrophenyl)methanone=O-acetyl oxime (photopolymerization initiator of formula (4))
[0058] Synthesis was carried out in the same manner as in Example 1. by 1 H-NMR (CDCl 3 ) when carrying out the identification of gained pure product compound, show following result.
[0059] δ=8.92(s, 1H), 8.49(d, 1H), 8.47(d, 2H), 8.14(s, 1H), 7.96(d, 1H), 7.61(d, 2H), 7.48(d, 1H) , 7.46(d, 1H), 4.30(q, 2H), 2.12(s, 3H), 1.90-1.83(m, 2H), 1.43-1.35(m, 2H), 1.35-1.23(m, 4H), 0.86 (t,3H)
[0060] In addition, a photosensitive composition was produced and evaluated in the same manner as in Example 1, and the exposure amount required for photohardening was 26 mJ / cm 2 .
Embodiment 3
[0062] (9-(2-ethylhexyl)-6-nitrocarbazol-3-yl)(4-nitrophenyl)methanone=O-acetyl oxime (photopolymerization initiator of formula (5))
[0063] Synthesis was carried out in the same manner as in Example 1. by 1 H-NMR (CDCl 3 ) when carrying out the identification of gained pure product compound, show following result.
[0064] δ=8.93(s, 1H), 8.46(d, 2H), 8.41(d, 1H), 8.15(s, 1H), 7.95(d, 1H), 7.60(d, 2H), 7.48(d, 1H) , 7.45(d, 1H), 4.17(q, 2H), 2.16(s, 3H), 2.07(m, 1H), 1.45-1.30(m, 4H), 1.30-1.26(m, 4H), 0.92(t ,3H), 0.86(t,3H)
[0065] In addition, a photosensitive composition was produced and evaluated in the same manner as in Example 1, and the exposure amount required for photohardening was 26 mJ / cm 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com