Process to produce fibrillar proteins and method of treating using fibrillar proteins
A fibrous protein, protein technology, applied in the preparation method of peptide, peptide/protein composition, animal/human protein, etc., can solve the problem of long time and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Materials and methods
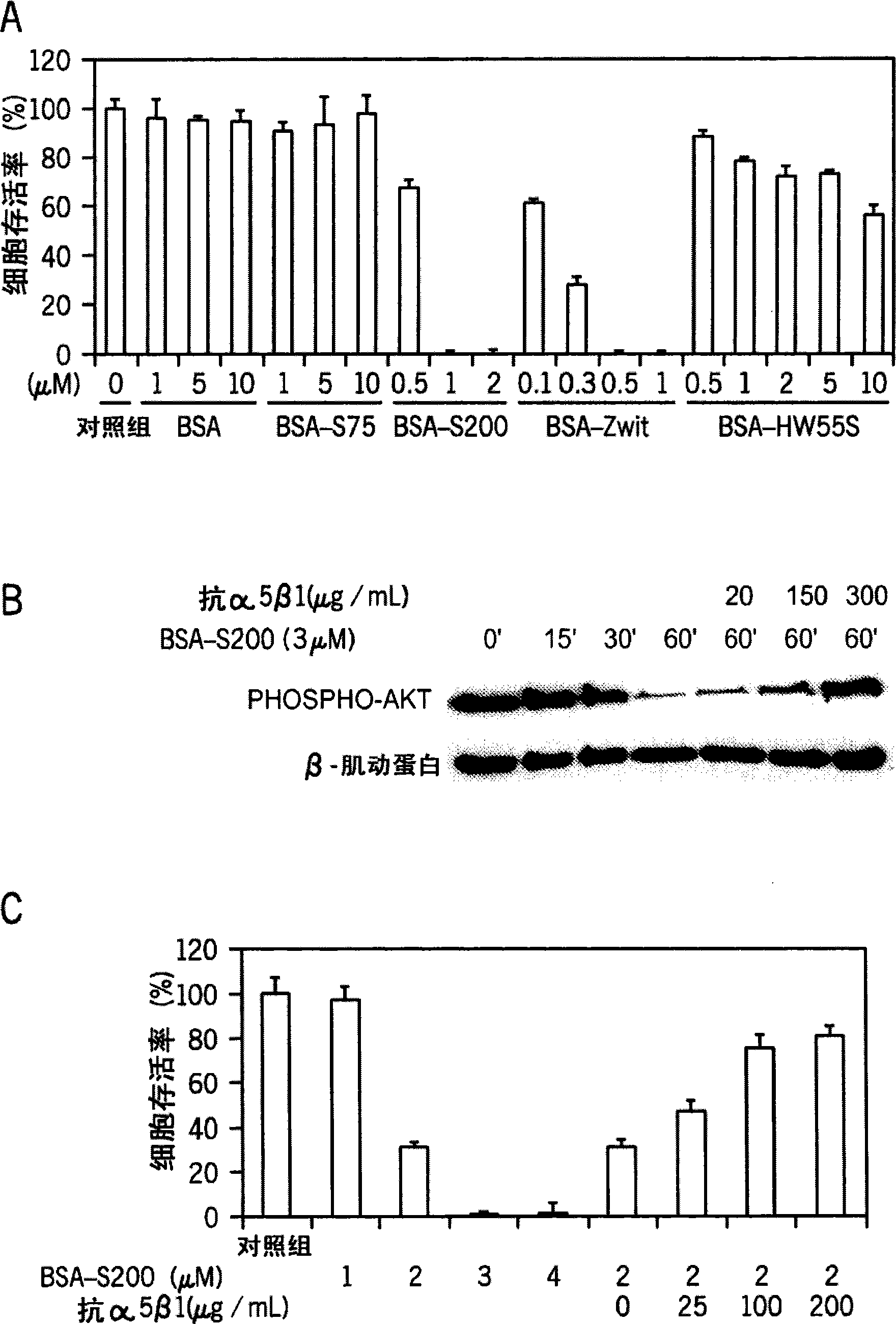
[0054] Material: Phospho-Ser 473 Akt antibody was purchased from Cell Signaling Technology. Zwittergent 3-14 was purchased from Calbiochem. Fibronectin (Fibronectin, FN), anti-actin antibody (anti-actin antibody), anti-integrin α5β1 polyclonal antibody (anti-integrin α5β1 polyclonal antibody) (function inhibitory antibody), horseradish peroxidase-conjugated antibody Mouse IgG secondary antibodies (horseradish peroxidase-coupled anti-mouse IgG secondary antibo dies), horseradish peroxidase-conjugated anti-rabbit IgG secondary antibodies (horseradish peroxidase-coupled anti-rabbit IgG secondary antibodies) and MTT test group were purchased from Chemicon International Corporation. Bovine serum albumin (BSA) was purchased from Bio Basic. Thioflavin T (Thioflavin, ThT) and sodium dodecyl sulfate (SDS) were purchased from Sigma.
[0055] Expression and purification of recombinant proteins VP1 and VP3. VP1 and VP3 are the capsid protein components of fo...
Embodiment 2
[0088] Materials and methods
[0089] Material: Phosphorus-Try 576 / 577 FAK(phospho-Try 576 / 577 FAK), phosphorus-Ser 473 Akt(phospho-Ser 473 Akt) and phosphorus-Ser 9 GSK-3β(phospho-Ser 9 GSK-3β) antibody was purchased from Cell Signaling Technology (Beverly, MA, USA). Phosphorus-Tyr 397 FAK(phospho--Tyr 397 FAK) antibodies were purchased from Biosource (Camerillo, California, USA). Zwittergent 3-14 was purchased from Calbiochem (San Diego, California, USA). Integrin α5β1 protein, anti-β-actin antibody, anti-integrin α5 antibody, anti-integrin α5β1 antibody (anti-integrin α5β1 antibody) ) (Functional inhibitory antibody), horseradish peroxidase-coupled anti-mouse IgG secondary antibodies, horseradish peroxidase-coupled anti-mouse IgG secondary antibodies, horseradish peroxidase-conjugated anti-rabbit IgG secondary antibodies (horseradish peroxidase -coupled anti-rabbit IgG secondary antibodies) and the MTT test set were purchased from Chemicon (Timekla, California). Anti-BSA an...
Embodiment 3
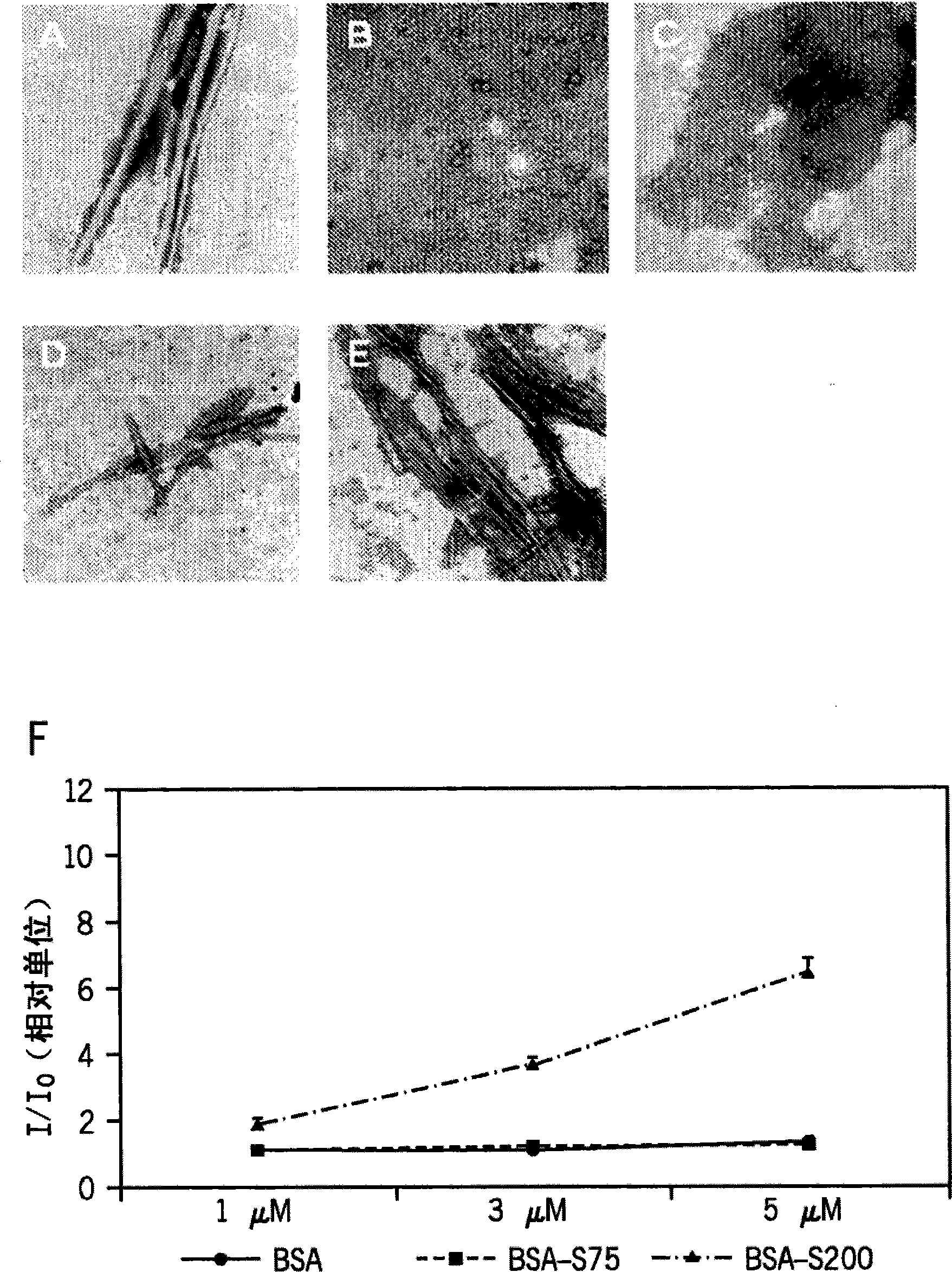
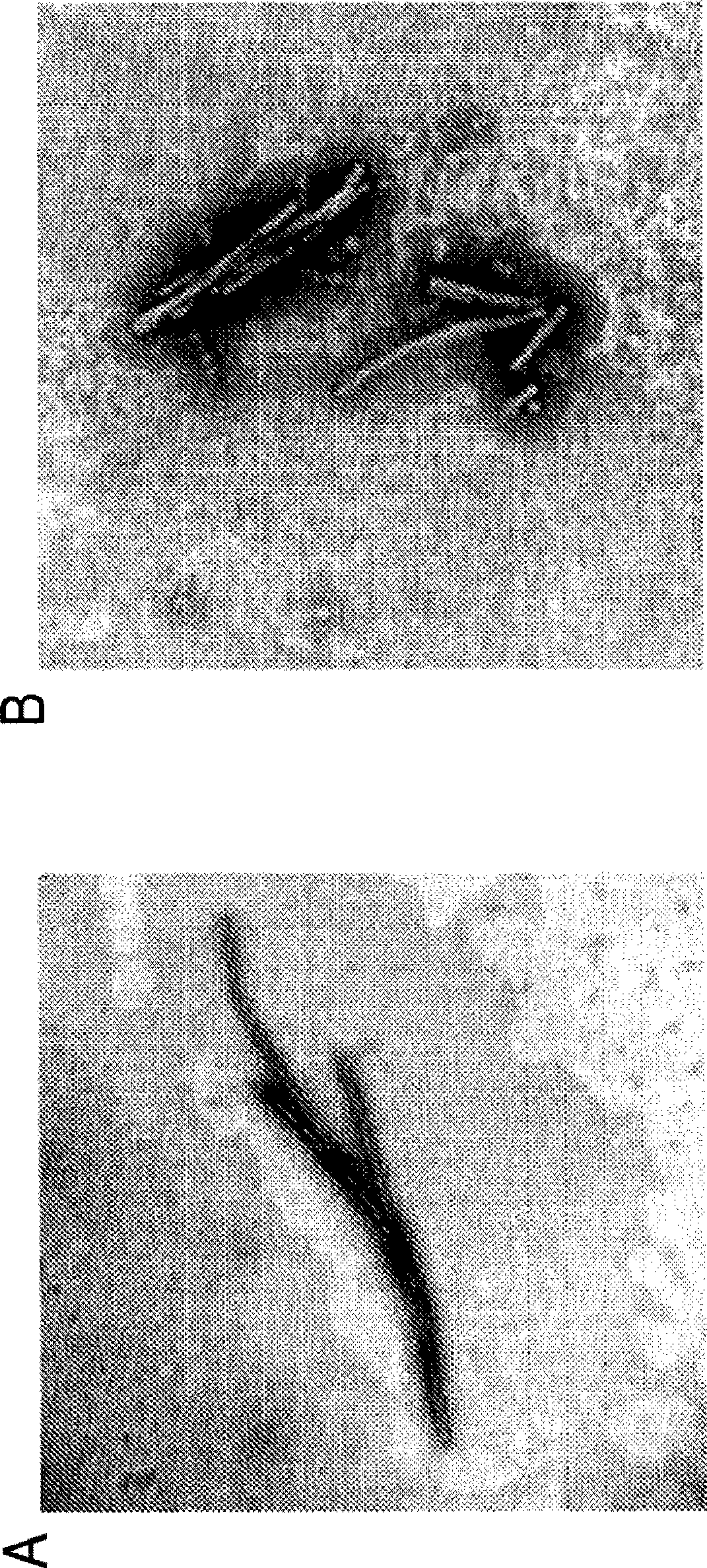
[0110] Previous experiments found that the use of Superdex-75 in the presence of 8M urea for bovine serum albumin can induce unfolded bovine serum albumin to have a fibril form; the recombinant protein VP1 also has the same situation, with fibril formation. This result can be determined by the increase in ThT ( Figure 8 ) And cytotoxic ( Picture 9 ) And confirmed. The use concentration of urea is not limited to 8M, and other molar ratios can also promote protein unfolding to the same or lesser extent.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com