Tetraacetyl talose compound and preparation method thereof
A technology for tetraacetyl talose and compounds, which is applied in the field of tetraacetyl talose compounds and their preparation, can solve the problems of few applied research, complex synthesis methods, and low talose content, and achieve simple separation and preparation methods Simple, easy-to-control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
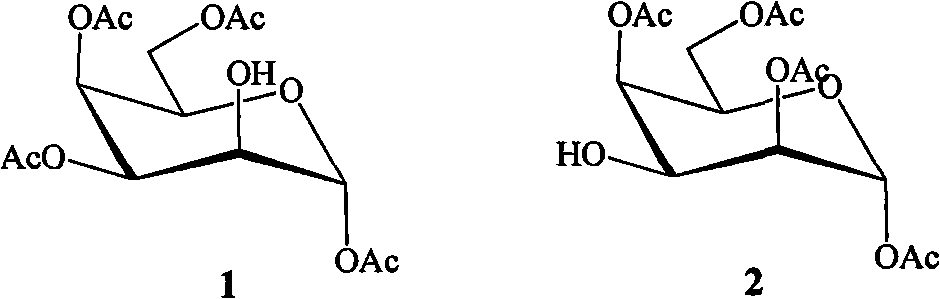
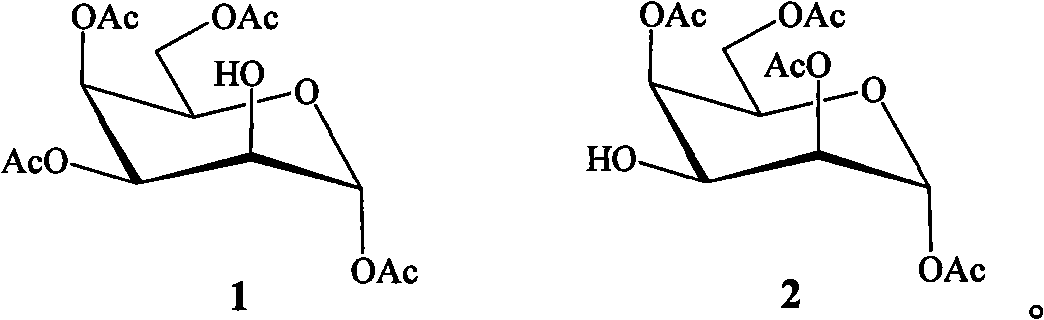
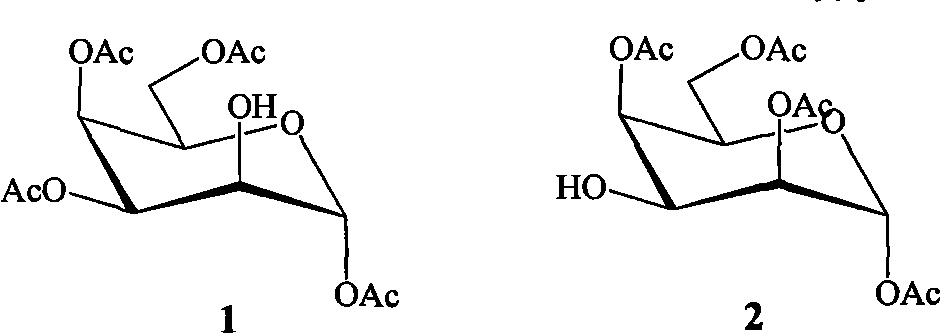
[0015] The preparation method of bicyclic alcohol glycoside compound of the present invention comprises the following steps:
[0016]
Embodiment 1
[0017] Embodiment 1, the synthesis of intermediate 3:
[0018] Mix D-galactose (18.0g, 100mmol), sodium acetate (9.0g, 110mmol), and 72ml of acetic acid, and control the temperature at about 120°C. After the reaction is completed, pour the reaction solution into 500ml of ice cubes, stir to precipitate a white solid, petroleum Ethyl ether acetate was recrystallized to obtain 36.5 g of a white solid with a yield of 93.6%.
Embodiment 2
[0019] Embodiment 2, the synthesis of intermediate 4:
[0020] Dissolve β-pentaacetylgalactopyranose (7.8g, 20mmol) in 90% trifluoroacetic acid aqueous solution, react at room temperature for 6.0h, evaporate the solvent after the reaction is completed, add isopropyl ether to precipitate a white solid, and recrystallize to obtain a white crystal 4.6 g, the yield was 66.0%. , to 1,3,4,6-tetra-acetylgalactopyranose.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com