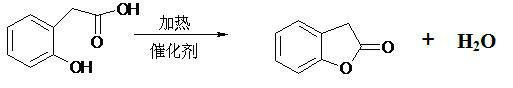
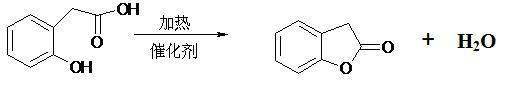
Production method of benzofuranone
A technology of benzofuranone and its production method, which is applied in the direction of organic chemistry, can solve the problems of large investment in factory equipment, difficult recovery of catalysts, environmental problems, etc., and achieve the effects of reducing product loss, reducing equipment investment, and simplifying post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Put 200 g o-hydroxyphenylacetic acid, 500 mL toluene and 2 g D001 macroporous strongly acidic cation exchange resin (produced by Shanghai Huizhi Resin Factory) into a 1000 mL reaction bottle, start stirring, heat to reflux, and separate the generated water in the water separator. After about 6 hours, when the content of o-hydroxyphenylacetic acid is lower than 1%, it is cooled, centrifugally filtered, and the filtered resin is reused. Toluene was removed from the filtrate to obtain 179 g of benzofuranone with a content of 96.5% and a yield of 98.0%.
Embodiment 2
[0022] Put 200 g of o-hydroxyphenylacetic acid, 500 mL of cyclohexane and 10 g of 001*7 (732) strongly acidic cation exchange resin (produced by Shanghai Huizhi Resin Factory) into a 1000 mL reaction bottle, start stirring, heat to reflux, and The resulting water is separated. When the content of o-hydroxyphenylacetic acid is lower than 1%, it is cooled, centrifugally filtered, and the filtered resin is reused. Toluene was removed from the filtrate to obtain 178 g of benzofuranone with a content of 96.5% and a yield of 97.4%.
Embodiment 3
[0024] Put 200 g of o-hydroxyphenylacetic acid, 500 mL of chlorobenzene and 2 g of 00X7 (732) cation exchange resin (Changzhou Tehua Trading Co., Ltd.) into a 1000 mL reaction bottle, start stirring, heat to reflux, and separate the generated in the water separator water. When the content of o-hydroxyphenylacetic acid is lower than 1%, it is cooled, centrifugally filtered, and the filtered resin is reused. Toluene was removed from the filtrate to obtain 177 g of benzofuranone with a content of 97% and a yield of 97.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com