Tyrosine kinase inhibitor and medicinal application thereof in treating malignant tumor
A technology for malignant tumors and medicinal salts, applied in the field of tyrosine kinase inhibitor compounds, can solve problems such as liver toxicity and side effects of imatinib, and achieve the effects of less side effects, less dosage, and reduced oxidative metabolism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
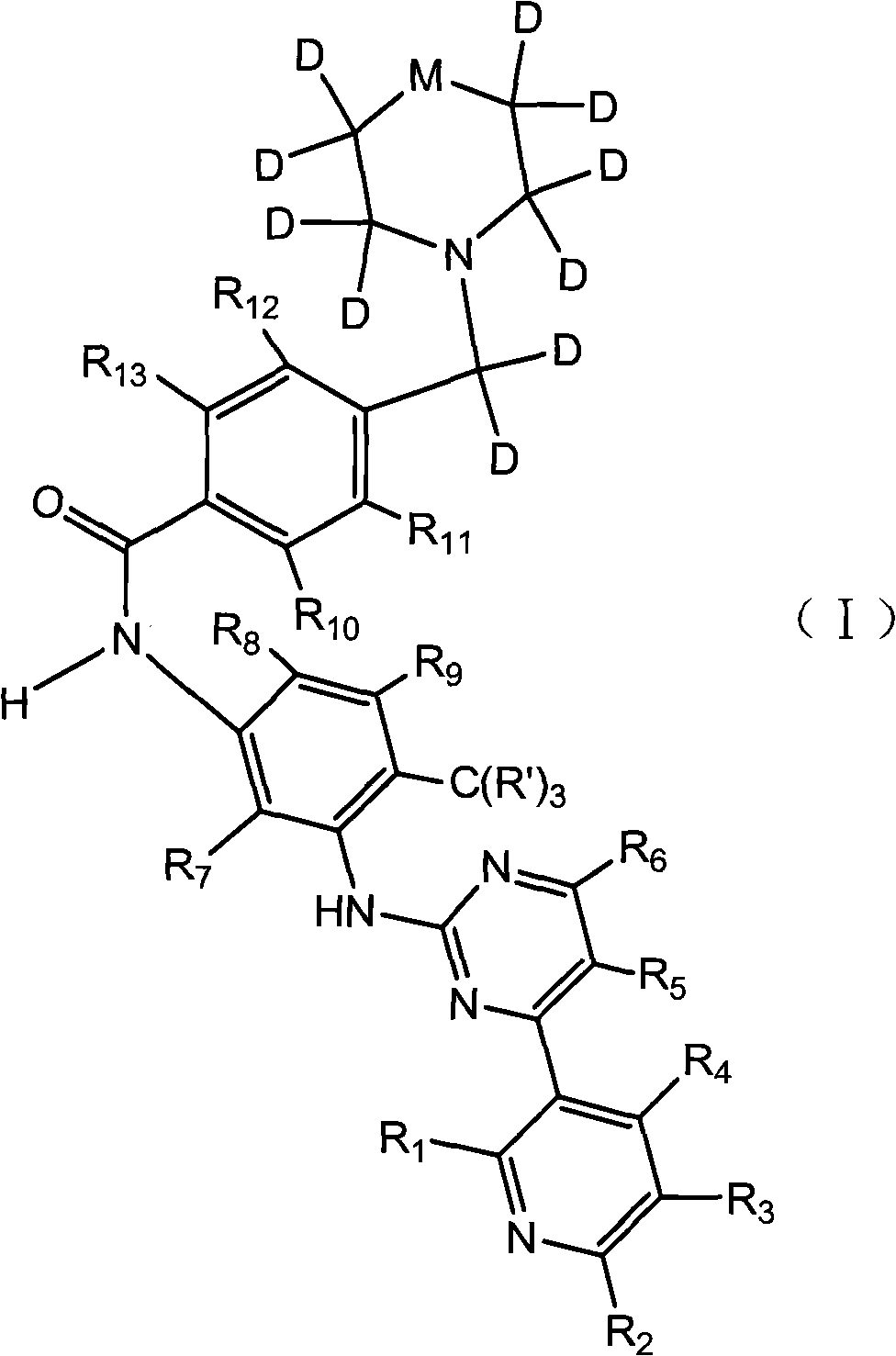
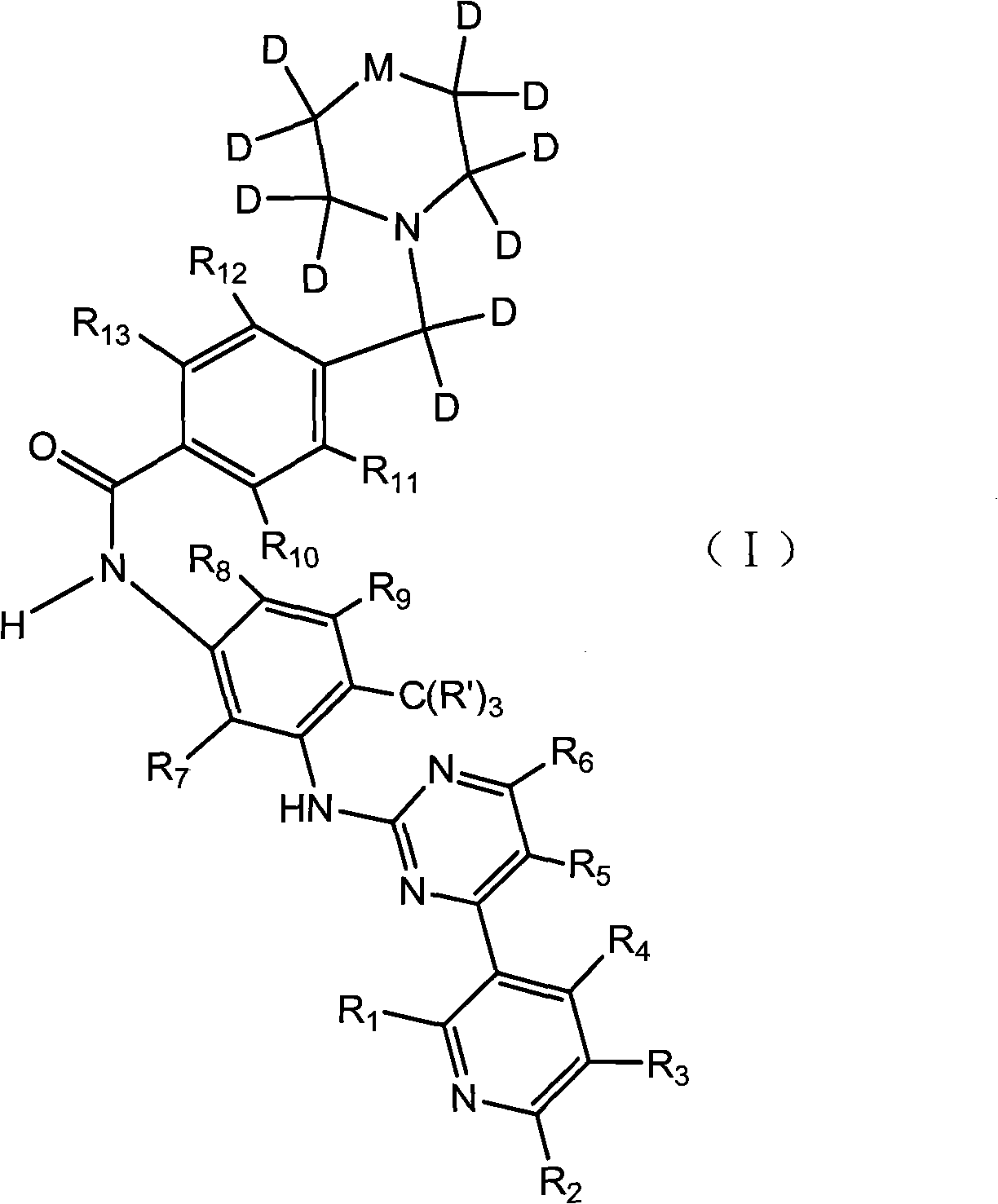
[0017] A tyrosine kinase inhibitor, the chemical name is: 1-trideuteromethyl-4-(4-(4-methyl-3-(4-(pyridine-3-ester) pyrimidine-2-ester amine) )phenylaminocarbonyl)-dideuteriobenzyl)-2,2,3,3,5,5,6,6-octadeutero-1-oxo-piperazine-6-methyl-N-1 -(4-(Pyridine-3-ester)pyrimidine-2-ester)benzene-1,3-diamine. Its structural formula is as follows:
[0018]
[0019] Above-mentioned compound can be prepared by following steps:
[0020] (1) At room temperature, add potassium carbonate to the stirred mixture of 2,6-piperazinedione, deuterium oxide and deuterated methanol, stir for 18 hours, concentrate to dryness, and dissolve the residue in ethyl acetate , washed successively with dilute hydrochloric acid (1M), saturated sodium bicarbonate solution, water and saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain 3,3,5,5-tetradeuterium-2,6-piperazine di ketone.
[0021] (2), at room temperature, add deuterated methyl iodide to the stirred mixture o...
Embodiment 2
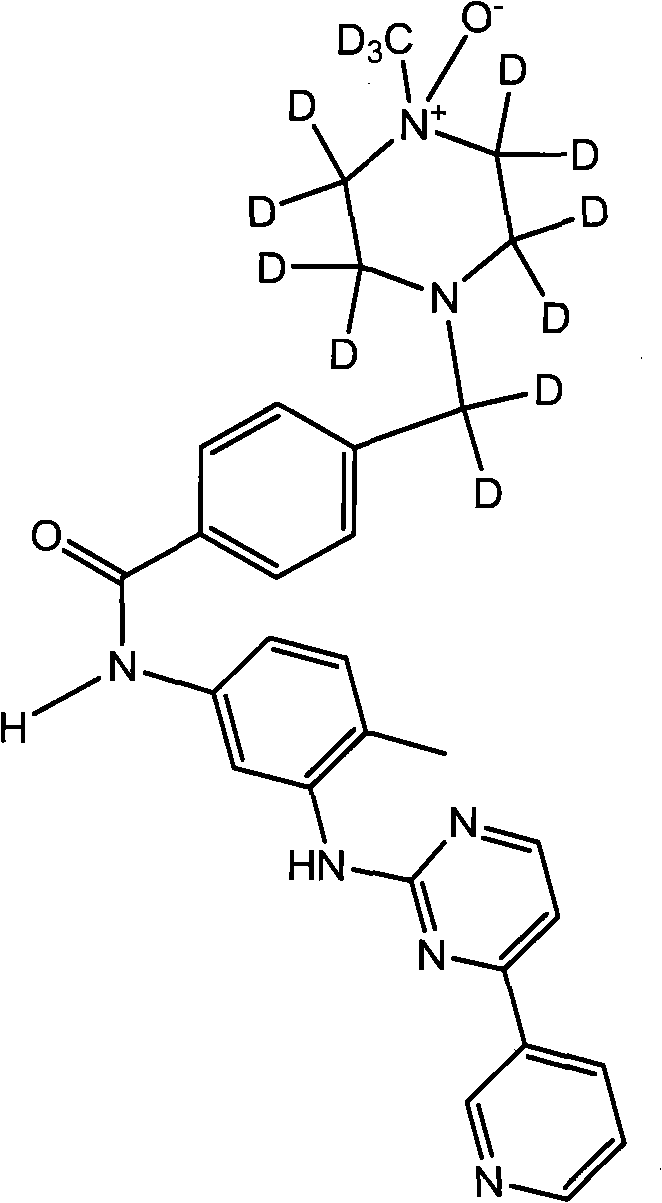
[0034] According to the tyrosine kinase inhibitor of this embodiment, the chemical name is: 4-((4-methoxy)-2,2,3,3,5,5,6,6-octadeutero-piperazine -1-ester)-dideuteromethyl)-N-(4-methyl-3-(4-(pyridine-3-ester)pyrimidine-2-esteramine)phenyl)benzamide. Its structural formula is as follows:
[0035]
[0036] Above-mentioned compound can be prepared by following steps:
[0037] (1), add m-chloroperoxybenzoic acid in the stirred mixed liquid of 4-trideuteromethyl-3,3,5,5-tetradeuterium-2,6-piperazinedione and dichloromethane, stir After the reaction, it was concentrated to dryness. The residue was dissolved in ethyl acetate and washed successively with dilute hydrochloric acid (1M), saturated sodium bicarbonate solution, water and saturated brine. Dry over anhydrous sodium sulfate, filter, and concentrate to give 4-hydroxy-3,3,5,5-tetradeuterium-2,6-piperazinedione (Intermediate 2-1).
[0038] (2) At room temperature, add methyl iodide to the stirred mixture of intermediate 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com