Method for preparing two types of super-hydrophobic membranes simultaneously by utilizing nickel chloride
A technology of nickel chloride and superhydrophobic, applied in the process of producing decorative surface effects, gaseous chemical plating, manufacturing microstructure devices, etc., can solve problems such as no related reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
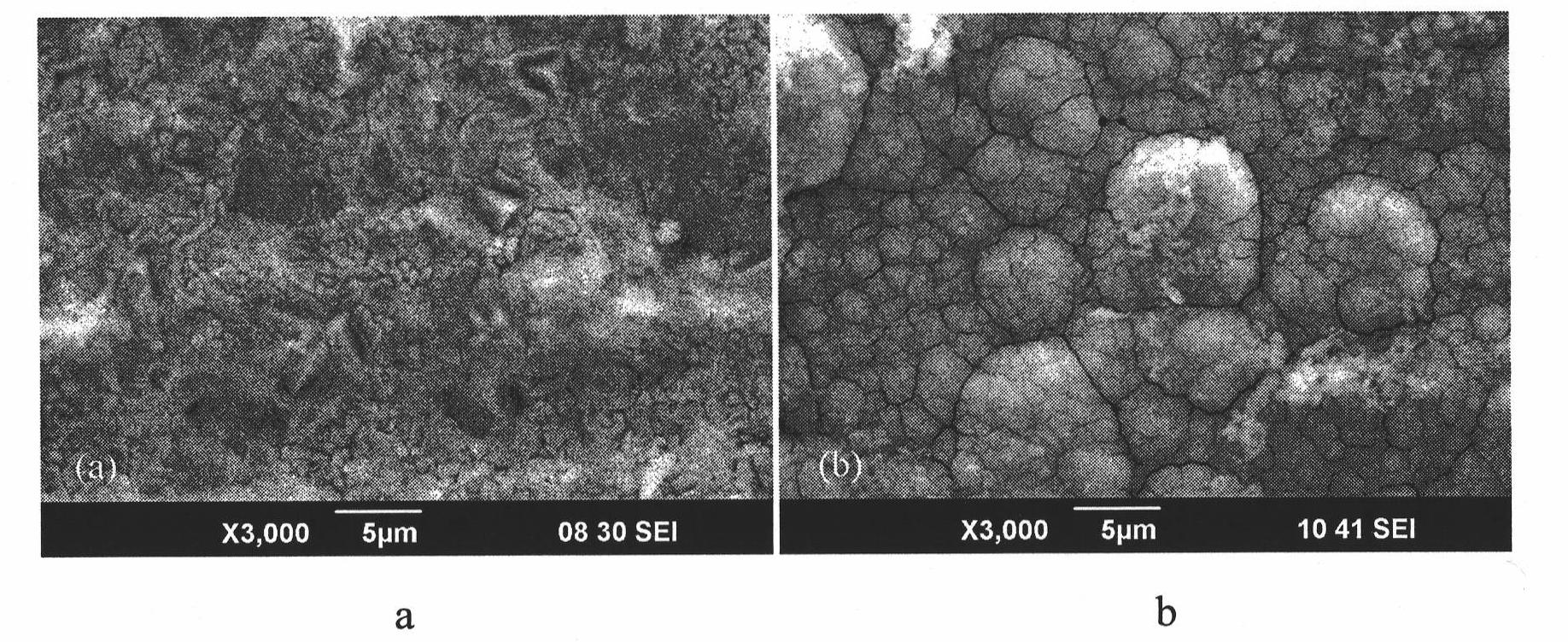
[0023] First 2.64g NiCl 2 ·6H 2 O is placed in 150ml of absolute ethanol to dissolve it completely to form a uniform solution; secondly, 2.741g of myristic acid powder is dispersed in the above solution under the condition of magnetic stirring, and mixed evenly to form a solution containing 0.08mol / l myristic acid electrolyte. Then put two pieces of 60mm×25mm×1.5mm copper sheets that have been polished and dried with 400 and 800 grit water sandpaper in advance, and put them in the electrolytic tank, and pour the above electrolyte into the electrolytic tank. Electrolysis, the superhydrophobic film formed on the anode and cathode after electrolysis for 15min see figure 1 , see Table 1 for specific contact angles.
Embodiment 2
[0025] First 2.64g NiCl 2 ·6H 2 O is placed in 150ml of absolute ethanol to dissolve it completely to form a uniform solution; secondly, 2.741g of myristic acid powder is dispersed in the above solution under the condition of magnetic stirring, and mixed evenly to form a solution containing 0.08mol / l myristic acid electrolyte. Then put two pieces of 60mm×25mm×1.5mm copper sheets that have been polished and dried with 400 and 800 grit water sandpaper in advance, and put them in the electrolytic tank, and pour the above electrolyte into the electrolytic tank. Electrolysis, the superhydrophobic film formed on the anode and cathode after electrolysis for 30min see figure 2 , see Table 1 for specific contact angles.
Embodiment 3
[0027] First 2.64g NiCl 2 ·6H 2 O is placed in 150ml of absolute ethanol to dissolve it completely to form a uniform solution; secondly, 2.741g of myristic acid powder is dispersed in the above solution under the condition of magnetic stirring, and mixed evenly to form a solution containing 0.08mol / l myristic acid electrolyte. Then put two 60mm x 25mm x 1.5mm copper sheets that have been sanded and dried with 400 and 800 grit water sandpaper in advance and placed in the electrolytic tank, and the above electrolyte is poured into the electrolytic tank, under a DC voltage of 15V Electrolysis, the superhydrophobic film formed on the anode and cathode after electrolysis for 30min see image 3 , see Table 1 for specific contact angles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com