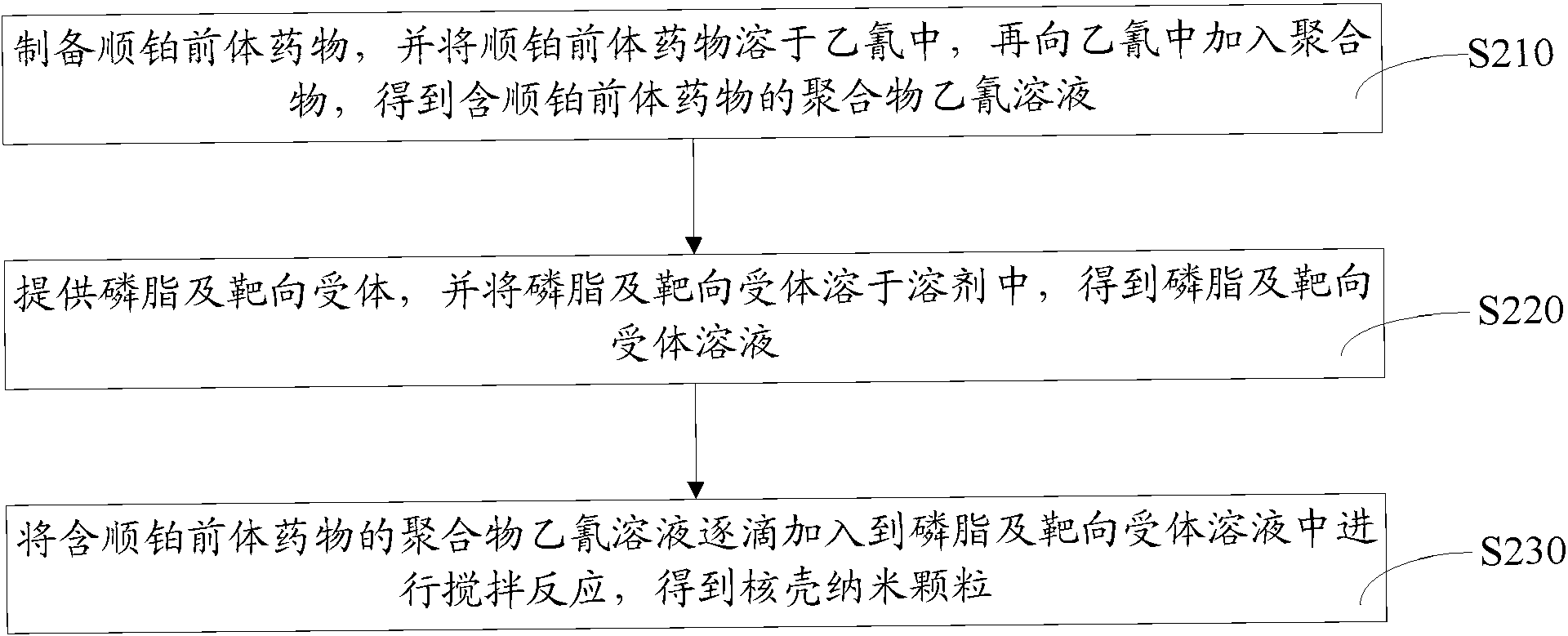
Cisplatin precursor medicine as well as preparation method thereof, and core-shell nano-particle as well as preparation method thereof
A prodrug and nanoparticle technology, applied in the field of nanomedicine, can solve the problems of difficult hydrophilicity, poor targeting, and weak stability, and achieve the effects of strong targeting, good compatibility, and easy use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
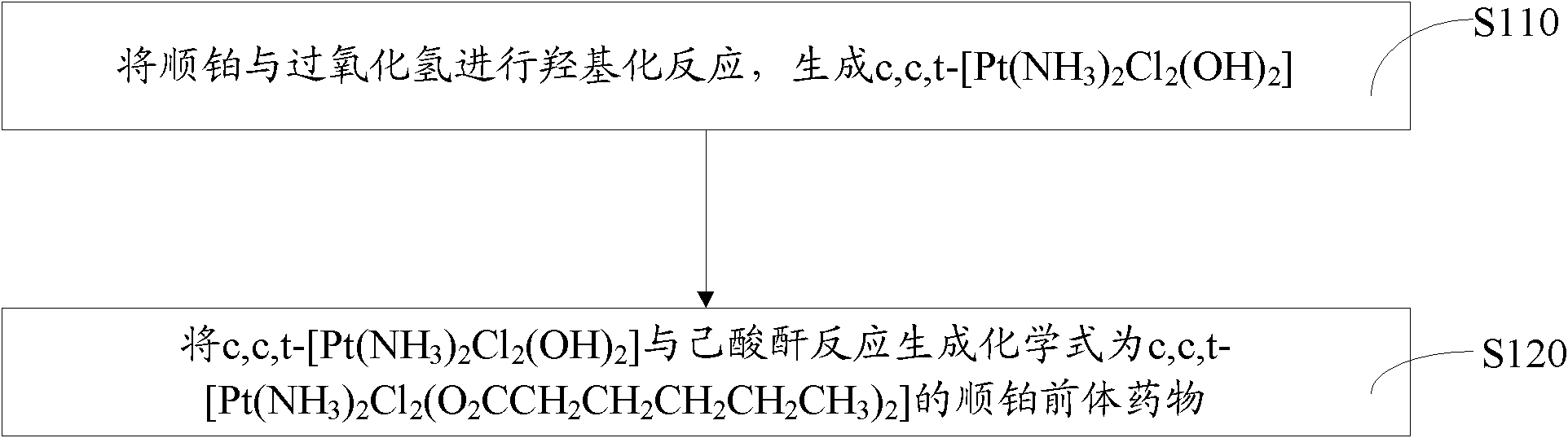
[0030] Such as figure 1 Shown, the preparation method of above-mentioned cisplatin prodrug, comprises the steps:
[0031] Step S110: carry out hydroxylation reaction of cisplatin and hydrogen peroxide to generate c, c, t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ]. details as follows:
[0032] Weigh a certain amount of cisplatin and dissolve it in distilled water, add 5 to 10 times the molar amount of cisplatin in H 2 o 2 , react at 40-60 degrees Celsius for 3-6 hours to generate c, c, t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ]. Preferably, after the reacted mixture is recrystallized, it is washed successively with cold distilled water, ethanol and ether, and the purified c, c, t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ].
[0033] Step S120: c, c, t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ] react with hexanoic anhydride to generate structural formula as c, c, t-[Pt(NH 3 ) 2 Cl 2 (O 2 CCH 2 CH 2 CH 2 CH 2 CH 3 ) 2 ] of cisplatin prodrugs. details as follows:
[0034] c, c, t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ] ...
Embodiment 1
[0057] Embodiment 1. Preparation of cisplatin prodrug:
[0058] Weigh 0.20 g of cisplatin and dissolve it in 5 mL of distilled water, add 5 times the molar amount of H 2 o 2 , after 3 hours of reaction at 50 ° C, a light yellow precipitate was produced, which was c, c, t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ]. After recrystallization, wash with cold distilled water, ethanol and ether successively, and dry to obtain purified c, c, t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ].
[0059] Weigh 0.70g c, c, t-[Pt(NH 3 ) 2 Cl 2 (OH) 2 ] dissolved in 10mL dimethyl sulfoxide, then added 0.90g hexanoic anhydride, stirred at room temperature for 24 hours, then added distilled water to precipitate a bright yellow precipitate, collected the precipitate by centrifugation, and washed the precipitate 3 times with ether to obtain purified c , c, t-[Pt(NH 3 ) 2 Cl 2 (O 2 CCH 2 CH 2 CH 2 CH 2 CH 3 ) 2 ].
Embodiment c
[0060] The present embodiment c, c, t-[Pt (NH 3 ) 2 Cl 2 (O 2 CCH 2 CH 2 CH 2 CH 2 CH 3 ) 2 ] with a yield of 43%. Product use 1 Characterized by H NMR, the solvent was DMSO-d 6, the chemical shifts are: 6.40-6.13 (S, 6H), 2.13-2.07 (t, J=8Hz, 4H), 2.13-2.07 (m, 4H), 1.25-1.19 (m, 4H), 1.17-1.09 ( m, 8H), 0.93-0.89 (t, J=8Hz, 6H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com