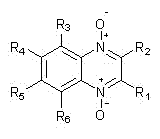
Quinoxaline double N-oxide derivative ligand and application thereof in promotion on copper-catalyzed C-O coupling reaction
A technology of quinoxaline bis-coupling reaction, applied in the field of new ligands, can solve the problems of long reaction time and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The structural formula of diphenyl ether is as follows:
[0041] Used in this example:
[0042] The aprotic polar solvent solvent is DMF;
[0043] Quinoxaline bis N -Oxide derivative ligand is 2-methoxycarbonyl-3-hydroxyquinoxaline bis N - oxides;
[0044] Catalyst copper salt is CuI;
[0045] The inorganic base reagent is Cs 2 CO 3 ;
[0046] The aryl halide is iodobenzene;
[0048] Add CuI (19.1 mg, 0.1 mmol, 10 mol%), 2-methoxycarbonyl-3-hydroxyquinoxaline bis N -Oxide (47.2 mg, 0.2 mmol, 20 mol%), Cs 2 CO 3 (812.5 mg, 2.5 mmol). The reaction flask was evacuated and flushed with argon. Add iodobenzene (0.112 mL, 1.0 mmol), phenol (0.134 mL, 1.5 mmol) and DMF (1.5 mL) under the protection of argon. The reactant was stirred at 100° C. for 12 h until the starting material was completely reacted (reaction monitored by TLC). After stopping the reaction, the obtained brown oily substance was diluted with ethyl acetate, filtered ...
Embodiment 2
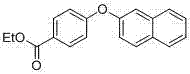
[0052] The structural formula of 2-phenoxynaphthalene is as follows:
[0053]
[0054] Used in this example:
[0055] The aprotic polar solvent solvent is DMF;
[0056] Quinoxaline bis N -Oxide derivative ligand is 2-methoxycarbonyl-3-hydroxyquinoxaline bis N - oxides;
[0057] Catalyst copper salt is CuI;
[0058] The inorganic base reagent is Cs 2 CO 3 ;
[0059] The aryl halide is iodobenzene;
[0060] Phenols are naphthalene-2-ols;
[0061] Add CuI (19.1 mg, 0.1 mmol, 10 mol%), 2-methoxycarbonyl-3-hydroxyquinoxaline bis N -Oxide (47.2 mg, 0.2 mmol, 20 mol%), Cs 2 CO 3 (812.5 mg, 2.5 mmol). The reaction flask was evacuated and flushed with argon. Add iodobenzene (0.112 mL, 1.0 mmol), naphthalene-2-ol (216.3 mg, 1.5 mmol) and DMF (1.5 mL) under the protection of argon. The reactant was stirred at 100° C. for 12 h until the starting material was completely reacted (reaction monitored by TLC). After stopping the reaction, the obtained brown oily substance was...
Embodiment 3
[0065] N The structural formula of -(4-phenoxyphenyl)acetamide is as follows:
[0066]
[0067] Used in this example:
[0068] The aprotic polar solvent solvent is DMF;
[0069] Quinoxaline bis N -Oxide derivative ligand is 2-methoxycarbonyl-3-hydroxyquinoxaline bis N - oxides;
[0070] Catalyst copper salt is CuI;
[0071] The inorganic base reagent is Cs 2 CO 3 ;
[0072] The aryl halide is iodobenzene;
[0073] Phenols are N -(4-hydroxyphenylphenyl)acetamide;
[0074] Add CuI (19.1 mg, 0.1 mmol, 10 mol%), 2-methoxycarbonyl-3-hydroxyquinoxaline bis N -Oxide (47.2 mg, 0.2 mmol, 20 mol%), Cs 2 CO 3 (812.5 mg, 2.5 mmol). The reaction flask was evacuated and flushed with argon. Add iodobenzene (0.112 mL, 1.0 mmol) under the protection of argon, N -(4-Hydroxyphenylphenyl)acetamide (226.7 mg, 1.5 mmol) and DMF (1.5 mL). The reactant was stirred at 100° C. for 15 h until the starting material was completely reacted (reaction monitored by TLC). After stopping th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com