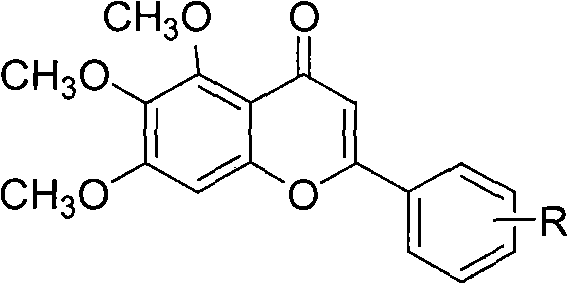
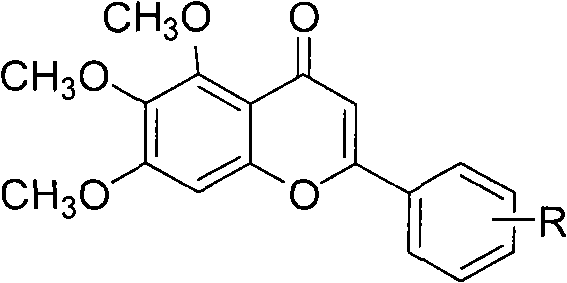
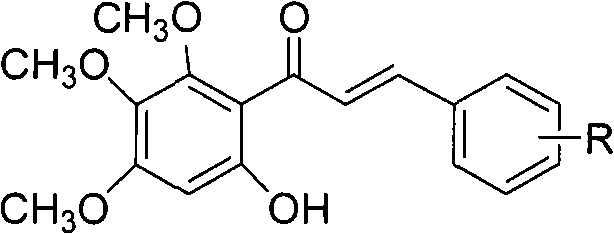
Method for compounding baicalein derivative
A technology of baicalein and derivatives, applied in the field of drug synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: 4'-methyl-5,6,7-trimethoxybaicalein (compound of formula I, R is 4'-methyl)
[0017] Add 17mL (150mmol) p-tolualdehyde, 19mL (200mmol) acetic anhydride, 10g potassium carbonate to the flask, and heat to reflux for 1.5h. It was extracted with ethyl acetate (3×250 mL), the organic layer was dried over anhydrous sodium sulfate, concentrated, and recrystallized from ethanol to obtain p-methylcinnamic acid (20 g, 82%).
[0018] Add 16.3g (100mmol) p-methylcinnamic acid, 80mL CH 2 Cl 2 , at N 2 Under protection, use an ice-water bath to maintain the temperature at 0-5°C, and drop 9.5mL (130mmol) SOCl 2 And a small amount of anhydrous DMF, react at 0-5°C for 2h. The solvent was distilled off under reduced pressure to obtain p-methylcinnamoyl chloride (13.5 g, 82%).
[0019] In the flask, add 13g (80mmol) p-methylcinnamoyl chloride, 12.9g (70mmol) 3,4,5-trimethoxyphenol, 80mL BF 3 -Et 2 O, heated to reflux for 30min, cooled to room temperature to precipitate p...
Embodiment 2
[0021] Example 2: Preparation of 4', 5,6,7-tetramethoxybaicalein (compound of formula I, R is 4'-methoxy)
[0022] Add 18.2mL (150mmol) p-methoxybenzaldehyde, 19mL (200mmol) acetic anhydride, 8g anhydrous potassium acetate to the flask, and heat to reflux for 2h. Add 15g of sodium carbonate to make the solution slightly alkaline, carry out steam distillation until the distillate has no oil droplets, add an appropriate amount of activated carbon and boil for 5-10min, suction filter while it is hot, add 30mL of concentrated hydrochloric acid to the filtrate to make the solution obviously acidic , a large amount of solids were precipitated, and p-methoxycinnamic acid (23 g, 85%) was obtained by suction filtration.
[0023] Add 17.8g (100mmol) p-methoxycinnamic acid, 80mL CH 2 Cl 2 , at N 2 Under protection, use an ice-water bath to maintain the temperature at 0-5°C, and drop 9.5mL (130mmol) SOCl 2 And a small amount of anhydrous DMF, react at 0-5°C for 2.5h. The solvent was ...
Embodiment 3
[0026] Example 3: Preparation of 3', 5,6,7-tetramethoxybaicalein (compound of formula I, R is 3'-methoxy)
[0027] Add 18.2mL (150mmol) m-methoxybenzaldehyde, 19mL (200mmol) acetic anhydride, 8g anhydrous potassium acetate to the flask, and heat to reflux for 3h. It was extracted with ethyl acetate (3×250 mL), the organic layer was dried over anhydrous sodium sulfate, concentrated, and recrystallized from ethanol to obtain m-methoxycinnamic acid (21.4 g, 80%).
[0028] Add 17.8g (100mmol) m-methoxycinnamic acid, 80mL CH 2 Cl 2 , at N 2 Under protection, use an ice-water bath to maintain the temperature at 0-5°C, and drop 9.5mL (130mmol) SOCl 2 and a small amount of anhydrous DMF, and reacted at 0-5°C for 3h. The solvent was distilled off under reduced pressure to obtain m-methoxycinnamoyl chloride (17 g, 80%).
[0029] In the flask, add 16.1g (80mmol) m-methoxycinnamoyl chloride, 12.9g (70mmol) 3,4,5-trimethoxyphenol, 80mL BF 3 -Et 2 O, heated to reflux for 1h, cooled t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com