Method for preparing (S)-4-hydroxide radical-2-oxo-1-pyrrolidine acetamide
A technology of pyrrolidine acetamide and oxo, applied in directions such as organic chemistry, can solve problems such as high cost, inability to provide products, and difficulty in recovery, and achieve the effects of short reaction cycle, improved yield, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
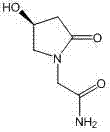
[0076] A kind of preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, its concrete steps are:
[0077] (a) Put 518.4g of glycinamide hydrochloride, 394g of sodium bicarbonate and 3.7L of absolute ethanol into a three-necked reaction flask, control the pH value to about 7.4, and heat up to reflux under stirring;
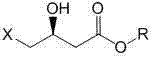
[0078] (b) After reflux for 2 hours, gradually add 781.6 g of (S)-4-chloro-3-hydroxybutyric acid ethyl ester dropwise, in the process of dropping, add the remaining sodium bicarbonate 394 g in 8 batches, and pass the pH value Check and control the amount of alkali added each time to ensure that the pH value of the reaction≤8.5;
[0079] (c) After (S)-4-halo-3-hydroxybutyrate was added dropwise, the reaction was refluxed for 24 hours, and the product (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide was determined by HPLC When the content is 75%, the reaction is terminated, and the obtained solution is hot filtered and concentrated to obtain the crude product ...
Embodiment 2
[0083] A kind of preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, its concrete steps are:
[0084] (a) Put 28.50 g of glycinamide hydrochloride, 20.65 g of sodium bicarbonate and 200 ml of absolute ethanol into a three-necked reaction flask, control the pH value to about 7.4, and heat up to reflux under stirring;
[0085](b) After 2 hours of reflux, 39.08 g of (S)-4-chloro-3-hydroxybutyric acid ethyl ester was gradually added dropwise. During the dropwise addition, the remaining sodium bicarbonate 20.65 g was added in 5 batches, and the pH value was passed. Check the amount of alkali added each time to ensure that the pH value of the reaction≤8.5;
[0086] (c) After (S)-4-halo-3-hydroxybutyrate was added dropwise, the reaction was refluxed for 24 hours, and the product (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide was determined by HPLC When the content was 74%, the reaction was terminated, and the obtained solution was concentrated by hot filtration to obtain...
Embodiment 3
[0090] A kind of preparation method of (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, its concrete steps are:
[0091] (a) Put 277.1g of glycinamide hydrochloride, 210.6g of sodium bicarbonate and 2000ml of absolute ethanol into a three-necked reaction flask, control the pH value to about 7.4, and heat up to reflux under stirring;
[0092] (b) After reflux for 2 hours, gradually add 417.8 g of (S)-4-chloro-3-hydroxybutyrate ethyl ester dropwise. During the dropping process, add the remaining sodium bicarbonate 210.6 g in 8 batches, and pass the pH value Check the amount of alkali added each time to ensure that the pH value of the reaction≤8.5;
[0093] (c) After (S)-4-halo-3-hydroxybutyrate was added dropwise, the reaction was refluxed for 24 hours, and the product (S)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide was determined by HPLC When the content was 72%, the reaction was terminated, and the obtained solution was concentrated by hot filtration to obtain the crude product of (S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com