Synthesis method of Formononetin
A technology for formononetin and a synthesis method, which is applied in directions such as organic chemistry, can solve the problems of severe reaction conditions, complex reaction products, expensive reagents and the like, and achieves the effects of high reaction efficiency, high application value, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
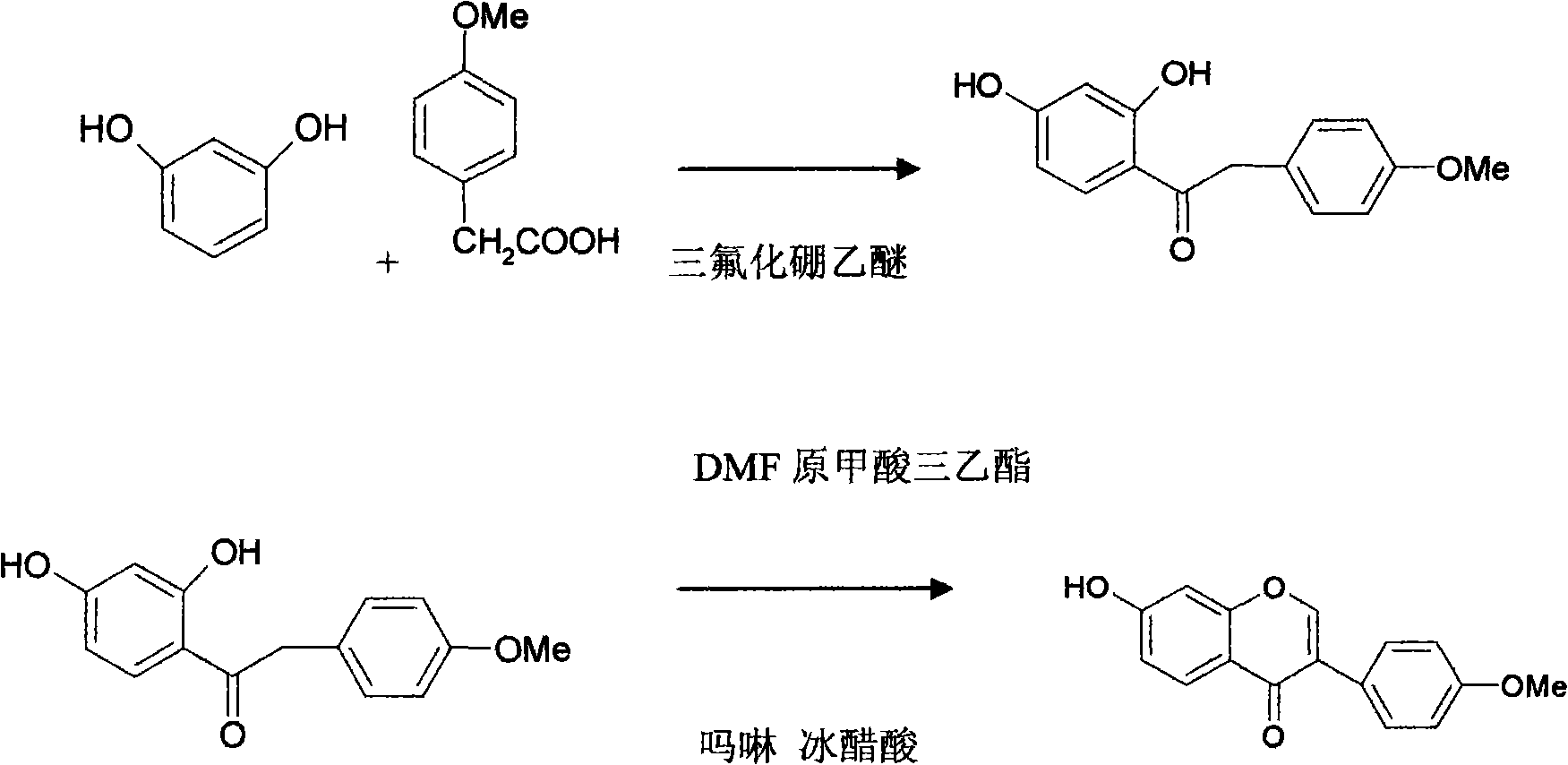
[0014] A synthetic method of formononetin, comprising the following steps:
[0015] The first step, condensation, in the drying reactor, add resorcinol, p-methoxyphenylacetic acid, boron trifluoride ether, the quality of resorcinol, p-methoxyphenylacetic acid, boron trifluoride ether The ratio is 1:1.5:2, at 80-90 ℃, the degree of vacuum is -0.08MPa, and the reaction is heated for 30min. After the reaction is completed, add water, the quality of the water is 1 times the total mass of the feed, and heat and reflux at 100-110 ℃ for 20min , the conventionally processed intermediates,
[0016] The second step, cyclization, in the dry reaction tank, add the intermediate, triethyl orthoformate, morpholine, DMF and glacial acetic acid, the mass ratio of the intermediate, triethyl orthoformate, morpholine, DMF and glacial acetic acid 1: 0.2: 0.2: 5: 0.3, separate by-product ethanol, heat under reflux for 6h, control the temperature to 110-135 ° C, after the reaction is completed, rec...
example 2
[0019] The first step, condensation, in the drying reactor, add resorcinol, p-methoxyphenylacetic acid, boron trifluoride ether, the quality of resorcinol, p-methoxyphenylacetic acid, boron trifluoride ether The ratio is 1:1.5:6, at 80-90 ℃, the vacuum degree is -0.08MPa, heating the reaction for 60min, after the reaction is completed, add water, the quality of the water is 3 times the total mass of the feeding , the conventionally processed intermediates,
[0020] The second step, cyclization, in the dry reaction tank, add the intermediate, triethyl orthoformate, morpholine, DMF and glacial acetic acid, the mass ratio of the intermediate, triethyl orthoformate, morpholine, DMF and glacial acetic acid 1: 0.2: 0.2: 10: 0.3, separate by-product ethanol, heat under reflux for 8h, control the temperature to 110-135 ° C, after the reaction is completed, recover DMF under reduced pressure, cool to 20 ° C, and add 5% of the mass of the intermediate to the residue. times the saturate...
example 3
[0023] A synthetic method of formononetin, comprising the following steps:
[0024] The first step, condensation, in the drying reactor, add resorcinol, p-methoxyphenylacetic acid, boron trifluoride ether, the quality of resorcinol, p-methoxyphenylacetic acid, boron trifluoride ether The ratio is 1:1.5:10, at 80-90 ℃, the degree of vacuum is -0.08MPa, heating and reacting for 90min, after the reaction is completed, add water, the quality of the water is 5 times the total mass of the feed, heat and reflux at 100-110 ℃ for 20min , cooled to precipitate crystallization, and the intermediate was obtained by conventional treatment,
[0025] The second step, cyclization, in the dry reaction tank, add the intermediate, triethyl orthoformate, morpholine, DMF and glacial acetic acid, the mass ratio of the intermediate, triethyl orthoformate, morpholine, DMF and glacial acetic acid 1: 0.5: 0.3: 8: 0.6, separate the reaction by-product ethanol, heat to reflux for 10h, control the temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com