Method for separating ginkgolide B from ginkgolide mixture
A technology of ginkgolides and mixtures, applied in the field of chemical engineering, can solve the problems of large equipment investment, high equipment requirements, and large solvent consumption, and achieve the effects of less consumption, low cost, and less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
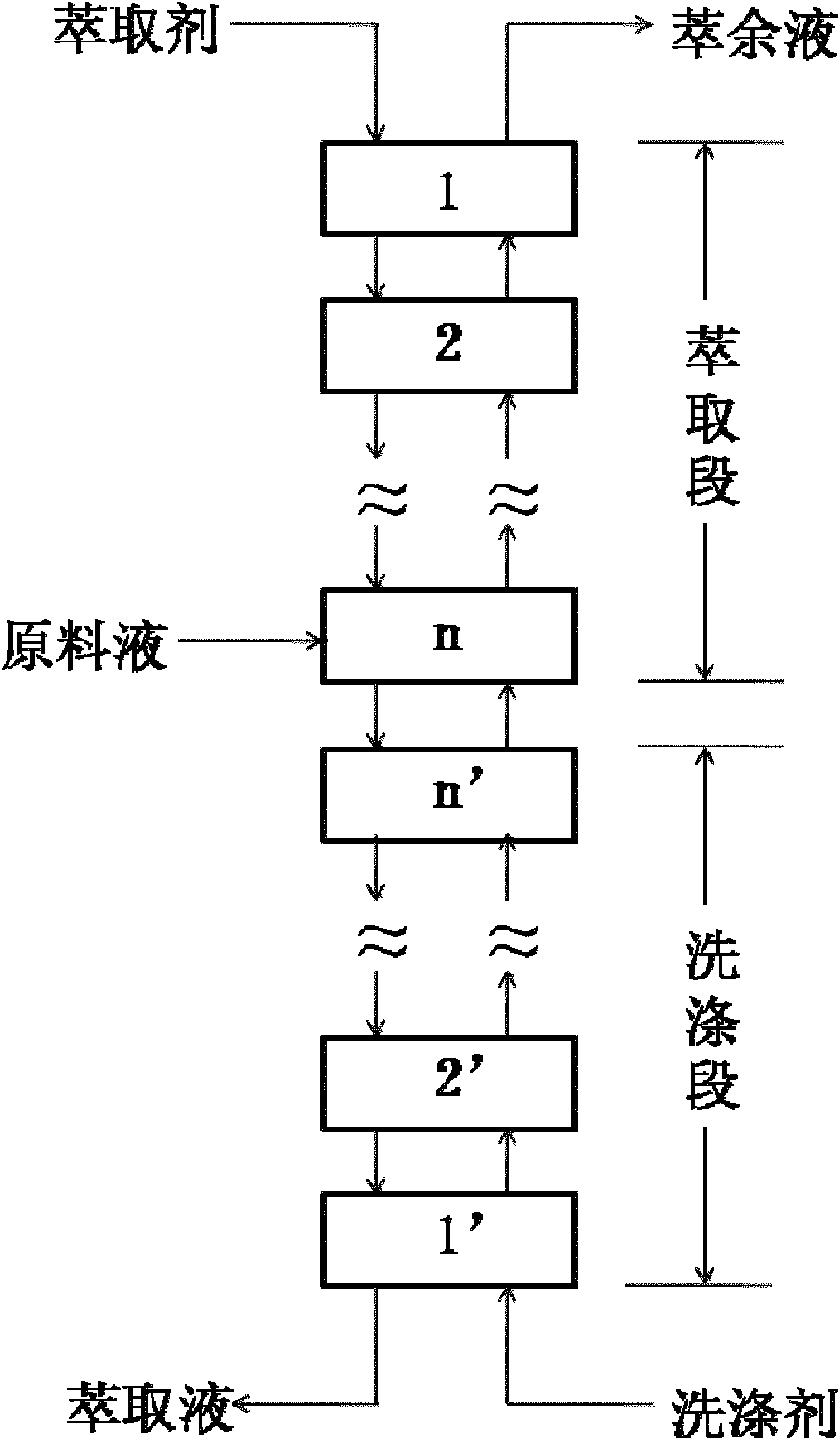
[0042] Commercially available ginkgolide mixture (wherein the percentage by weight of ginkgolide A, ginkgolide B, ginkgolide C is respectively 60%, 26% and 10%) and ethyl acetate is made into 20 g / liter stock solution , with N-ethylpyridinium bromide ([EPy]Br)-water mixed solvent as extractant ([EPy]Br weight fraction is 70%), with ethyl acetate as detergent, extractant, detergent, raw material solution The flow ratio of the three is 3:2.7:1. Fractional extraction is carried out in a fractional distillation extraction device at 40°C. The fractional distillation extraction is divided into an extraction section and a washing section. The last stage of the extraction section enters the fractionation extraction system, the detergent enters the fractionation extraction system from the first stage of the washing section, and the raw material liquid is combined into the extraction section at the last stage of the washing section, and the extraction phase and the washing phase are subj...
Embodiment 2
[0044] The commercially available ginkgolide mixture (wherein the total weight percentage of ginkgolide A, ginkgolide B, and ginkgolide C is 96%) and butyl acetate is made into a stock solution of 0.5 g / liter, and 1-butyl -3-Dimethylimidazolium tetrafluoroborate ionic liquid ([BMIm][BF 4 ]) as the extractant, with butyl acetate as the detergent, the flow ratio of the extractant, the detergent, and the raw material liquid is 2: 4.2: 1, and the fractionation extraction is carried out in a fractionation extraction device at 20°C, and the fractionation extraction is divided into The extraction section and the washing section, the extractant enters the fractionation extraction system from the first stage of the extraction section, the raw material liquid enters the fractionation extraction system from the last stage of the extraction section, and the detergent enters the fractionation extraction system from the first stage of the washing section. The last stage merges the raw mater...
Embodiment 3
[0046] Ginkgo biloba extract is subjected to steps such as general extraction, precipitation, evaporation solvent, and the total weight percentage that separates and obtains ginkgolide A, ginkgolide B, ginkgolide C is the solid of 42%, and the solid that obtains is mixed with n-butyl Alcohol is made into the stock solution of 100 g / liter, with tributylammonium chloride ([HNBu 3 ]Cl)-water mixed solvent is the extractant ([HNBu 3 ] Cl weight fraction is 95%) as extractant, with n-butanol as detergent, the flow ratio of extractant, detergent, raw material liquid three is 13: 1.7: 1, carries out fractionation in fractionation extraction device under 70 ℃ Extraction, fractional distillation extraction is divided into extraction section and washing section, the extractant enters the fractionation extraction system from the first stage of the extraction section, the raw material liquid enters the fractionation extraction system from the last stage of the extraction section, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com