Eukaryon expression vector and application thereof to preparing medicament for inhibiting leukemia cell proliferation
A technology of eukaryotic expression vectors and leukemia cells, applied in the field of genetic engineering, can solve the problems of time-consuming, labor-intensive, poor economy, and no biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
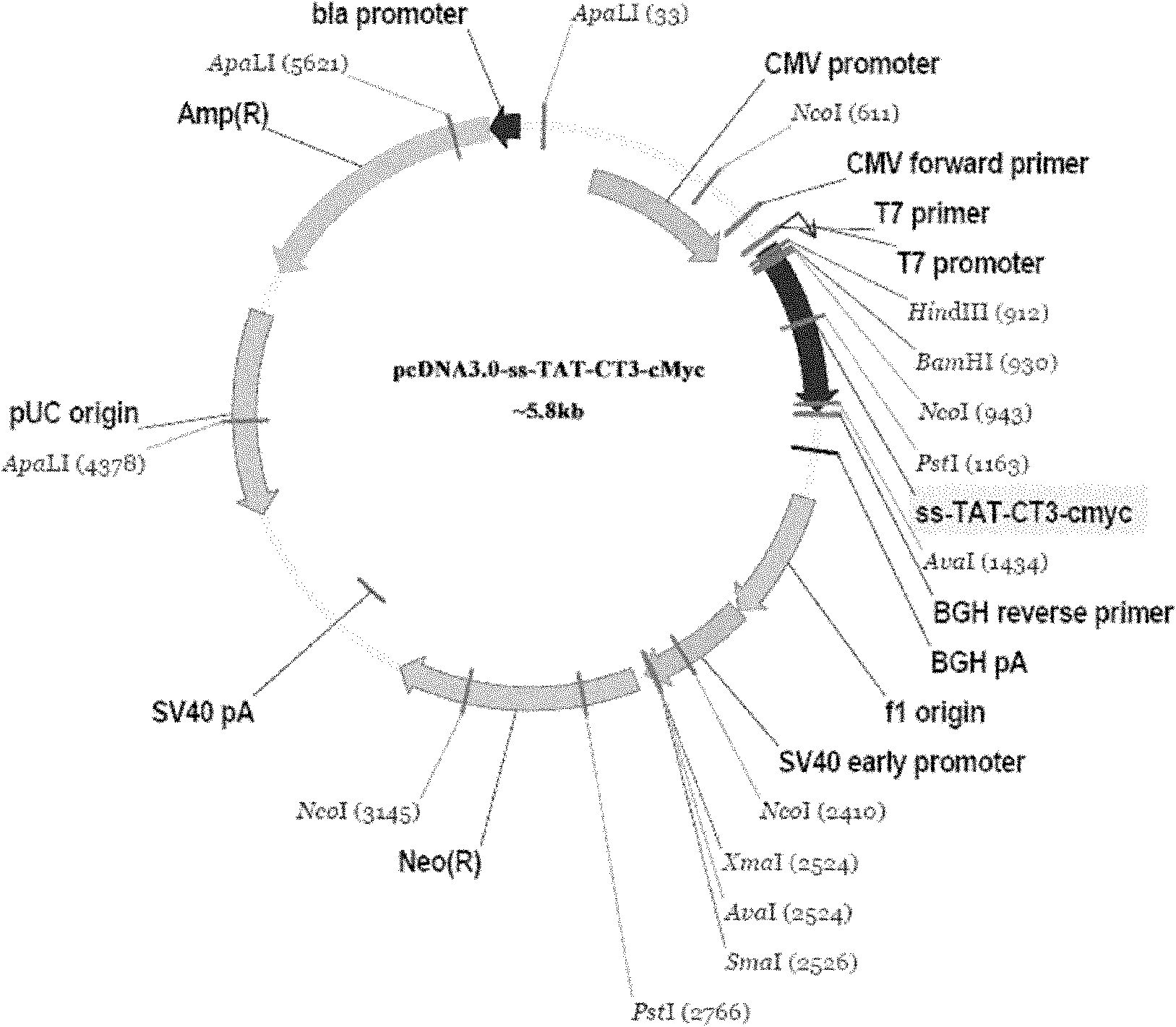
[0028] Example 1: Construction of pcDNA3.0-190CT3 eukaryotic expression vector
[0029] 1.1 Required reagents
[0030] Restriction enzymes Sal I, Nhe I, Xho I and BamH I were purchased from Shanghai Yingjun Company (Invitrogen, Shanghai). DNA polymerase, PCR product purification kit, and gel recovery kit were purchased from Takara Bioengineering Co., Ltd. (Takara, Dalian). The eukaryotic expression vector pcDNA3.0 was purchased from Invitrogen (Carlsbad, CA, USA). Mouse anti-human cMyc epitope (tag) monoclonal antibody was purchased from Santa Cruz Company (Santa Cruz, CA, USA); FITC-labeled fluorescent goat anti-mouse secondary antibody was purchased from Shanghai Kangcheng Company (Kangcheng, Shanghai). All the following primers were synthesized by Shanghai Yingjun (Invitrogen, Shanghai).
[0031] 1.2 Construction preparation of eukaryotic vector pcDNA3.0-190CT3
[0032] Using the eukaryotic expression vector pcDNA3.0-gp190CT3 as a template (see Chinese Patent ZL20041001...
Embodiment 2
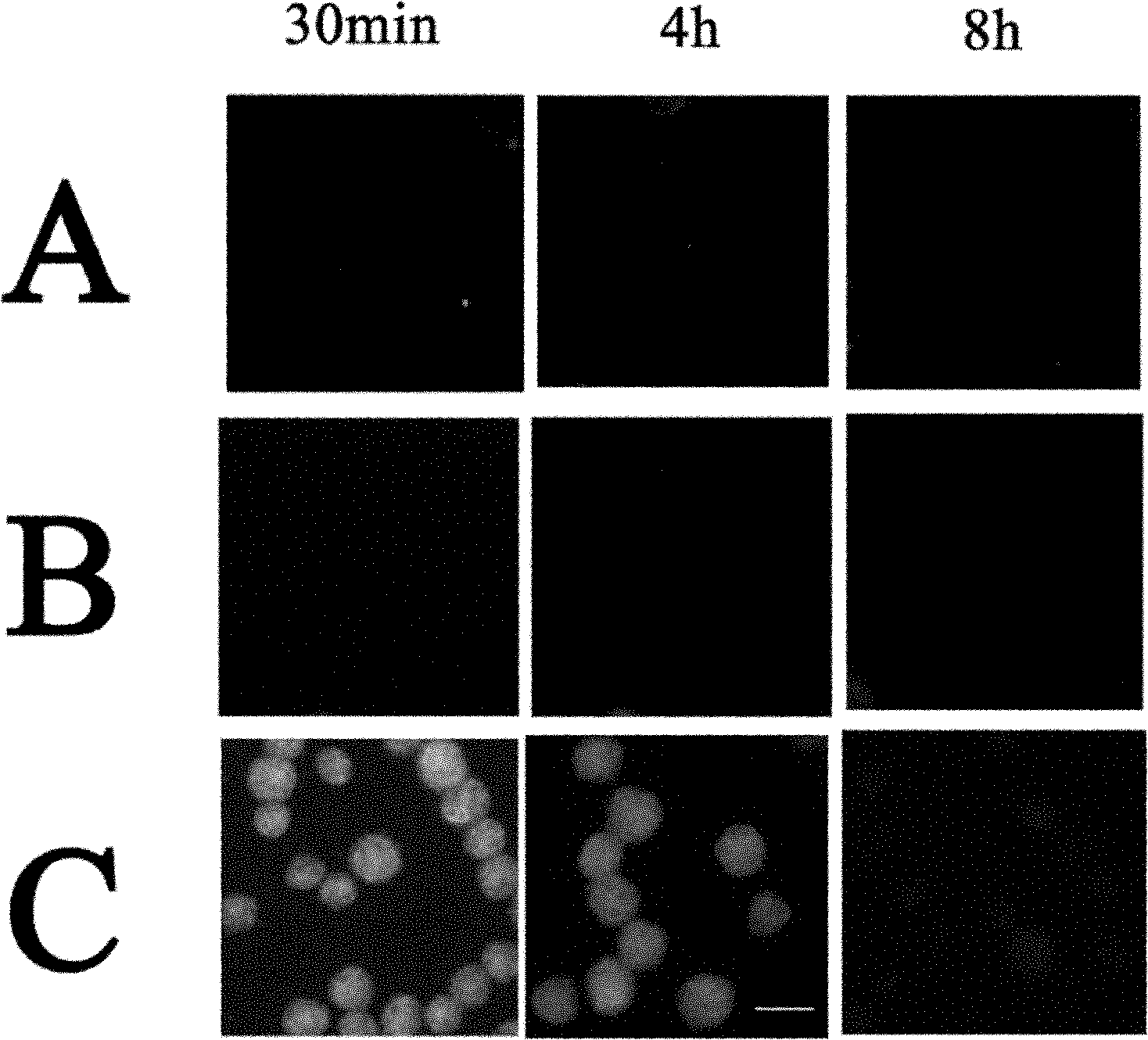
[0045] Example 2: Establishing a stable pcDNA3.0-190CT3 recombinant CHO cell line
[0046] First determine the appropriate concentration of G418 (Invitrogen) in the screening medium. The method is as follows: 1000 CHO cells per well are inoculated in a 24-well cell culture plate, and G418 is added to make the concentration respectively 500, 1000, 2000, and 3000 μg / ml. The wells in which the cells were completely killed in 10-14 days were used as the screening concentration of G418.
[0047] Transfect pcDNA3.0-190CT3 into CHO cells according to the instruction manual of the transfection reagent Fugene 6. 24 hours after transfection, the cells were subcultured at a ratio of 1:10, and the cultured cells were screened with pressure selection medium containing 3000 μg / ml G418. After 10 to 14 days, the neomycin-resistant cell clone colonies were surrounded by cloning rings. , Digested with trypsin and expanded in 96-well, 24-well and 6-well cell culture plates in turn. Different c...
Embodiment 3
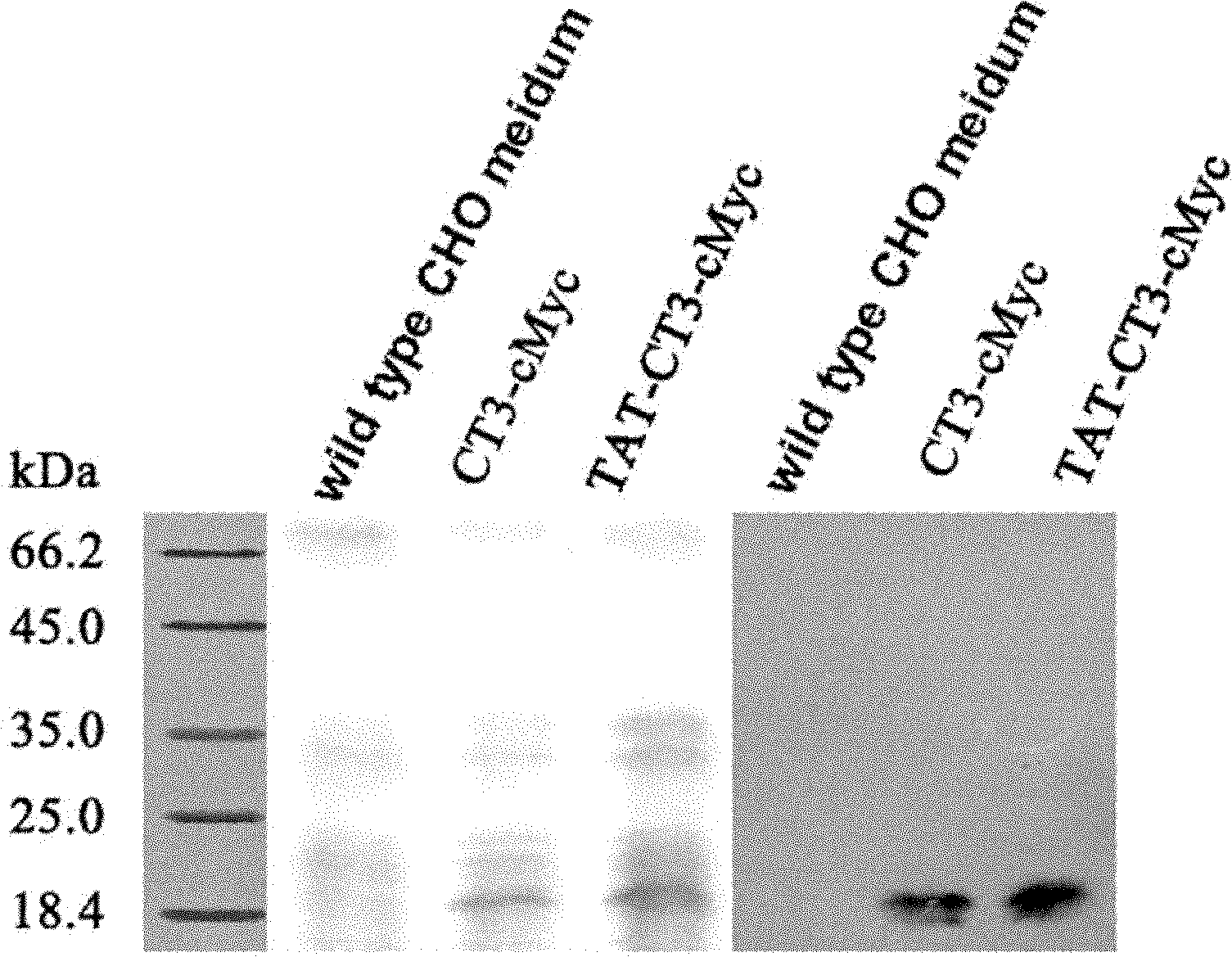
[0054] Example 3: Isolation and purification of fusion protein 190CT3
[0055] The above CHO-190CT3 cell line according to the following medium composition, in 75cm 2 Expand culture in a culture flask: 90% RPMI 1640 medium, 9% fetal bovine serum (FBS), 1% penicillin and streptomycin, and 3000 μg / ml G418 neomycin was added after passaged cells adhered to the wall. The culture condition is 37℃+5%CO 2 . When the cells are fused to 70-80%, replace the medium with 15ml of 100% RPMI 1640 medium. After 72 hours, the supernatant was collected, centrifuged at 10,000 rpm for 20 minutes to remove cell debris, and the remaining supernatant was immediately stored at -20°C. Repeat the above supernatant recovery procedure until the recovered volume reaches 1L. Before using the protein chromatography column to purify related proteins, put the supernatant at 4°C to let it melt slowly, and then pass through a 0.45 μm filter to remove possible impurities, dead cells and cell debris, ready to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com