Method for producing zinc hydrosulfite by using sodium hydrosulfite filtration residues
A technology of zinc dithionite and hydrosulfite, which is applied in the direction of thiosulfate/dithionite/polythionate, etc., can solve the problems of single use, inability to consume zinc powder waste residue, etc., and achieve raw material utilization High, less waste effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
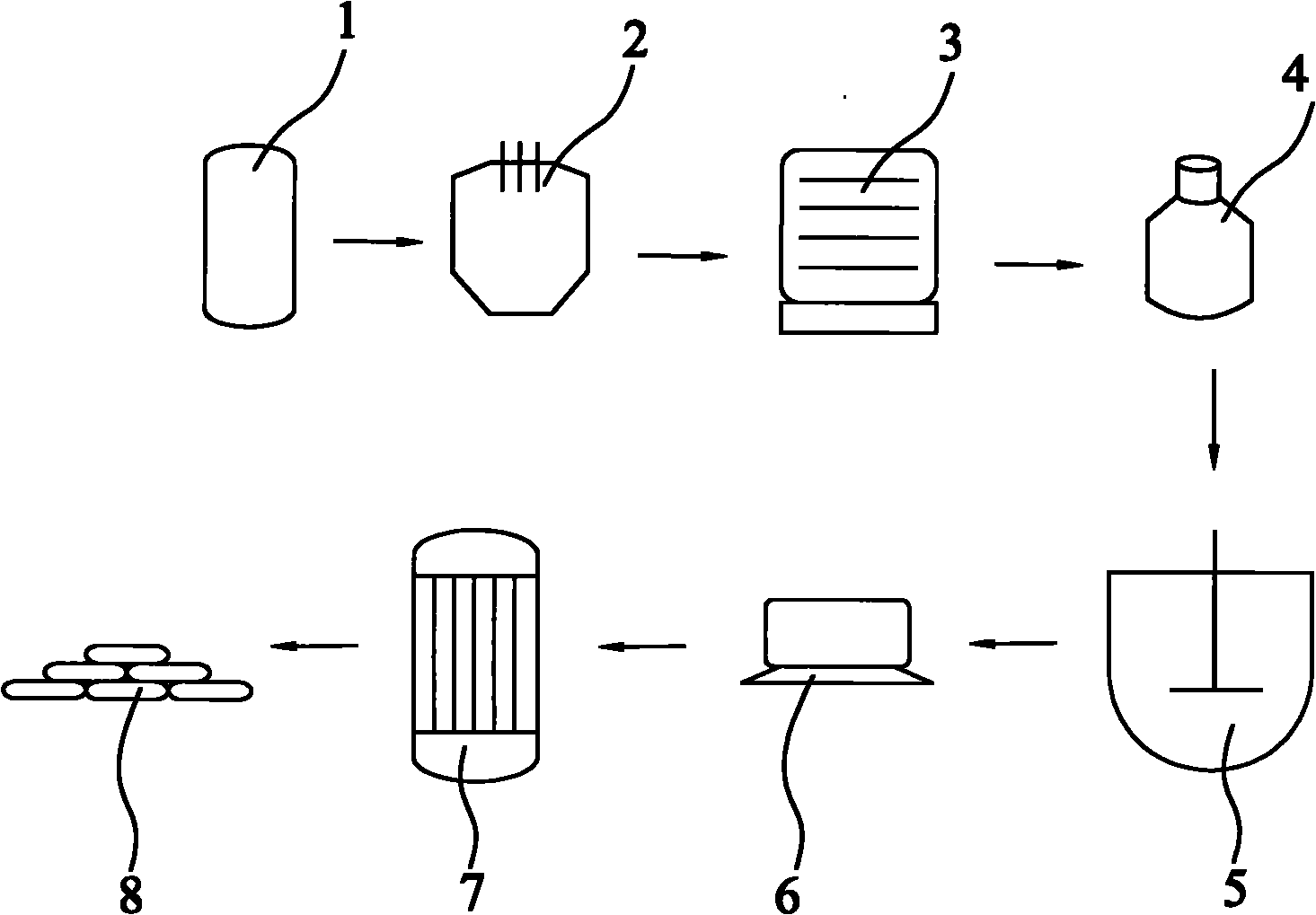
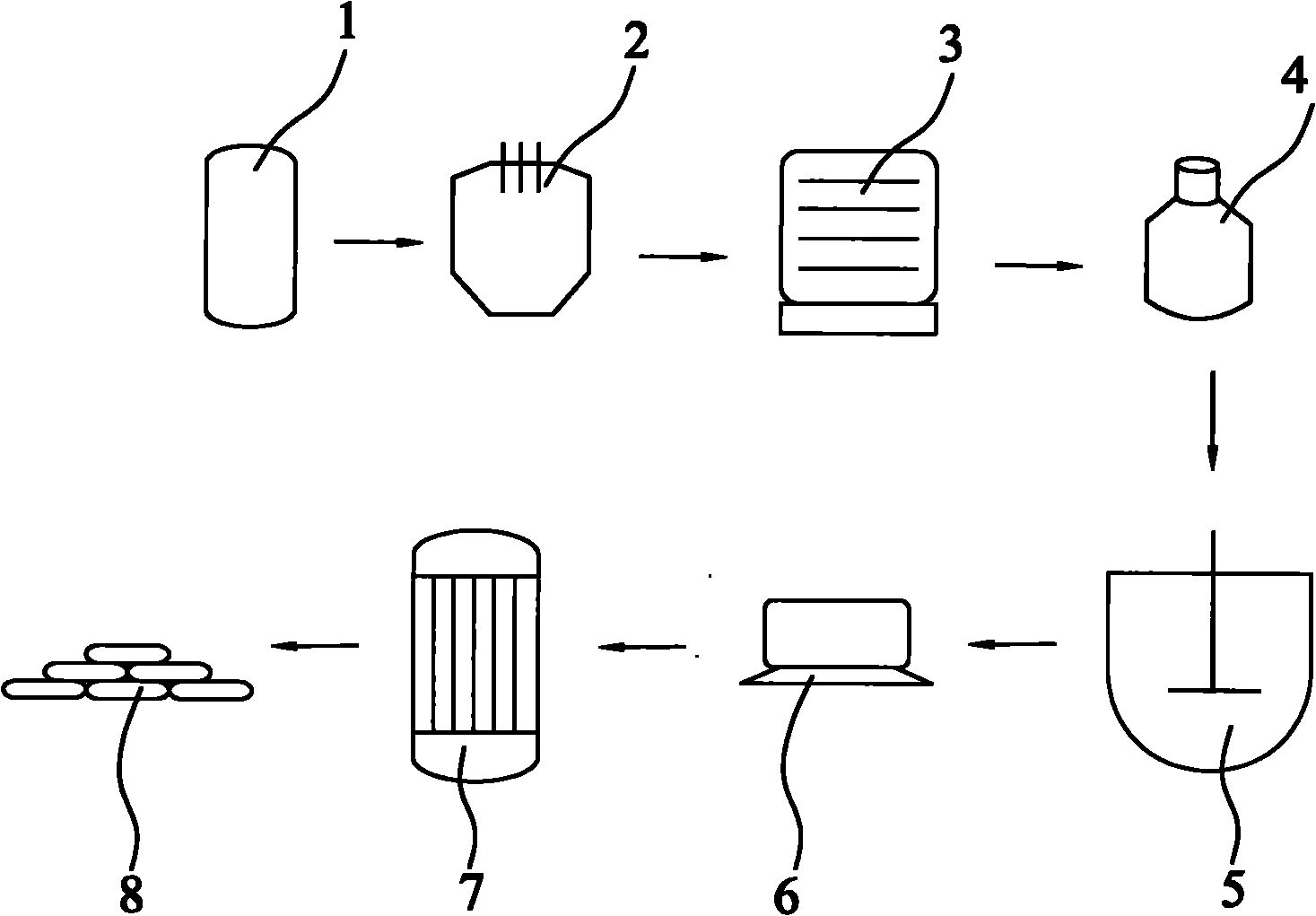
[0027] refer to figure 1 , a method for producing zinc dithionite with hydrosulfite filter residue, weighing 495kg of hydrosulfite waste residue, and converting the content of zinc hydroxide in the hydrosulfite waste residue to 297kg, after pulverization, it is heated to 300°C in a rotary kiln (1) at a high temperature, and then At the same time, zinc hydroxide starts dehydration reaction, and the zinc hydroxide in the sodium hydrosulfite waste residue is calcined into granular zinc oxide; meanwhile, the zinc sulfate in the sodium hydrosulfite waste residue has a pure substance content of 9.66kg in 495kg sodium hydrosulfite, and is decomposed into Zinc oxide and sulfur dioxide gas, so as to achieve the purpose of desulfurization; put the obtained zinc oxide and coke into the electric arc furnace (2) according to the pure mass ratio of 1:0.15, heat to 1300-1400 ° C, zinc oxide and carbon monoxide react to obtain Carbon dioxide gas and zinc vapor; Zinc vapor is condensed into zi...
Embodiment 2
[0029] refer to figure 1 , a method for producing zinc dithionite with hydrosulfite filter residue, weighing 495kg of hydrosulfite waste residue, and converting the content of zinc hydroxide in the hydrosulfite waste residue to 297kg, after pulverization, it is heated to 300°C in a rotary kiln (1) at a high temperature, and then At the same time, zinc hydroxide starts dehydration reaction, and the zinc hydroxide in the hydrosulfite waste residue is calcined into granular zinc oxide; meanwhile, the zinc sulfate in the hydrosulfite waste residue has a pure substance content of 9.66kg in 500kg hydrosulfite, and is decomposed into Zinc oxide and sulfur dioxide gas, so as to achieve the purpose of desulfurization; the obtained zinc oxide and coke are put into the electric arc furnace (2) according to the pure mass ratio of 1:0.1, heated to 1300-1400 ° C, and the zinc oxide and carbon monoxide react to obtain Carbon dioxide gas and zinc vapor; the zinc vapor is condensed into zinc p...
Embodiment 3
[0031] refer to figure 1, a method for producing zinc dithionite with hydrosulfite filter residue, weighing 495kg of hydrosulfite waste residue, and converting the content of zinc hydroxide in the hydrosulfite waste residue to 297kg, after pulverization, it is heated to 300°C in a rotary kiln (1) at a high temperature, and then At the same time, zinc hydroxide starts dehydration reaction, and the zinc hydroxide in the sodium hydrosulfite waste residue is calcined into granular zinc oxide; meanwhile, the zinc sulfate in the sodium hydrosulfite waste residue has a pure substance content of 9.66kg in 495kg sodium hydrosulfite, and is decomposed into Zinc oxide and sulfur dioxide gas, so as to achieve the purpose of desulfurization; put the obtained zinc oxide and coke into the electric arc furnace (2) according to the pure mass ratio of 1:0.20, heat to 1300-1400 ° C, zinc oxide and carbon monoxide react to obtain Carbon dioxide gas and zinc vapor; carbon dioxide reacts chemically...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com