Method for measuring roxithromycin
A technology of roxithromycin and roxithromycin tablets, which is applied in the direction of color/spectral characteristic measurement and analysis through chemical reaction of materials, can solve the problems that the determination of roxithromycin has not been reported yet, and reach the instrument price Inexpensive, good selectivity, high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
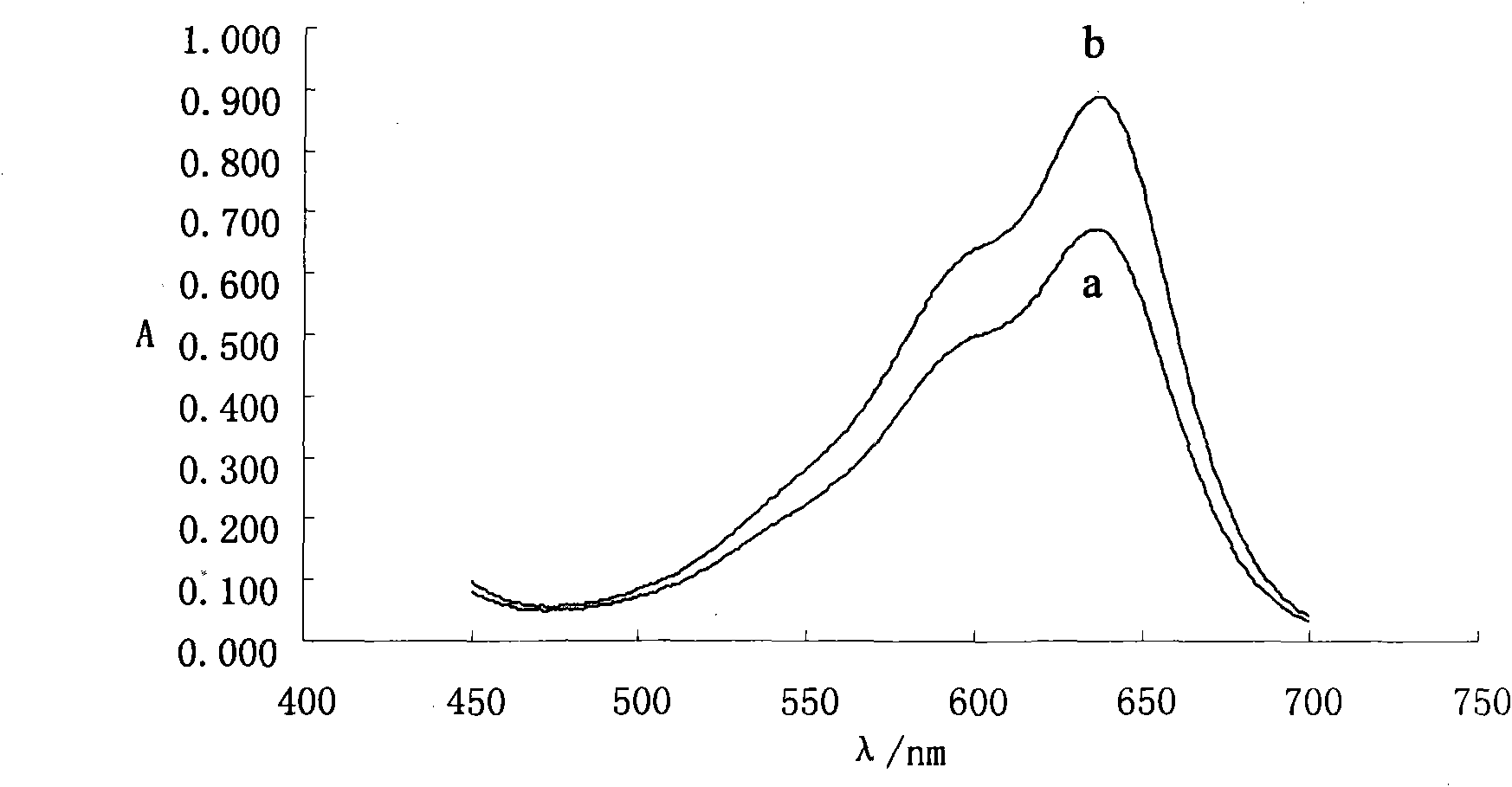
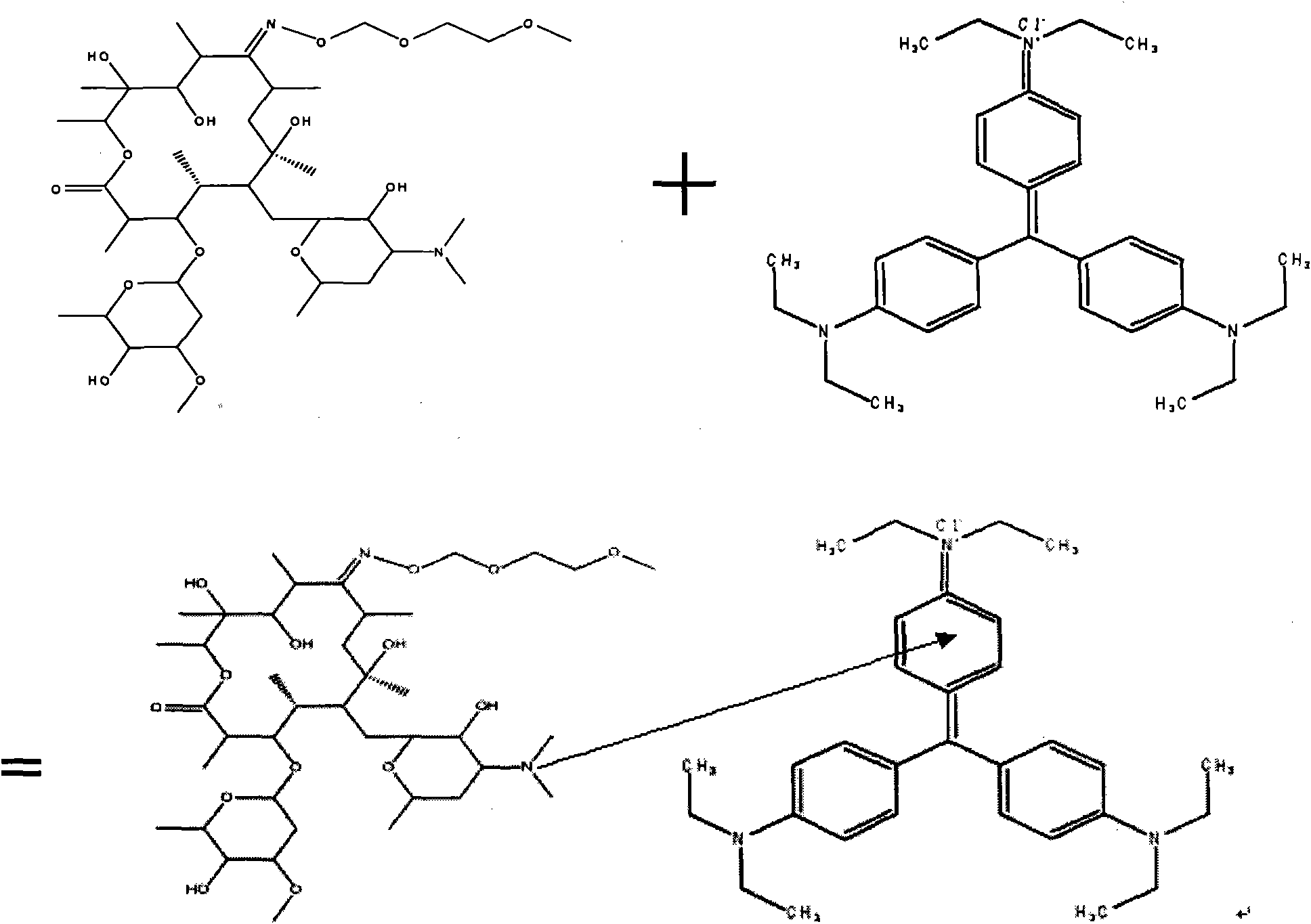
[0012] Add 0.0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0mL 0.52mg / L roxithromycin solution in turn to 10mL colorimetric tube, 1.5mL 5.067×10 -4 mol / L ethyl violet solution, then add 0.5mL 0.125mol / L HCl solution, dilute to the mark with twice distilled water, and shake well. Heat it in a water bath at 50°C for 10 minutes, take it out and cool it to room temperature, measure the absorbance of the roxithromycin solution at a wavelength of 637nm with a 1cm cuvette on a UV-visible spectrophotometer, using twice distilled water as a reference Value A 637nm and the absorbance value A of the reagent blank solution without roxithromycin 0 , to calculate the absorbance difference ΔA 637nm =A 637nm -A 0 . Its absorbance difference ΔA 637nm The linear regression equation with roxithromycin concentration C is ΔA 637nm =3.9904C+0.0065, correlation coefficient 0.9990, apparent molar absorptivity ε 637 =3.34×10 6 L·mol -1 cm -1 . Take another two roxithromycin tablets (marked amount 150mg / ta...
Embodiment 2
[0014] Add 0, 0.5, 1.0, 1.5, 2.0, 2.5, 3.0mL 0.52mg / L roxithromycin solution in turn to 10mL colorimetric tube, 1.5mL 5.067×10 -4 mol / L ethyl violet solution, then add 0.5mL0.125mol / L HCl solution, dilute to the mark with twice distilled water, and shake well. Heat it in a water bath at 50°C for 10 minutes, take it out and cool it to room temperature, measure the absorbance of the roxithromycin solution at a wavelength of 637nm with a 1cm cuvette on a UV-visible spectrophotometer, using twice distilled water as a reference Value A 637nm and the absorbance value A of the reagent blank solution without roxithromycin 0 , to calculate the absorbance difference ΔA 637nm =A 637nm -A 0 , the absorbance difference ΔA 637nm The linear regression equation with roxithromycin concentration c is ΔA 637nm =3.9904c+0.0065, correlation coefficient 0.9990, apparent molar absorptivity ε 637 =3.34×10 6 L·mol -1 cm -1 . Take another 3 capsules of roxithromycin capsules (marked amount 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com