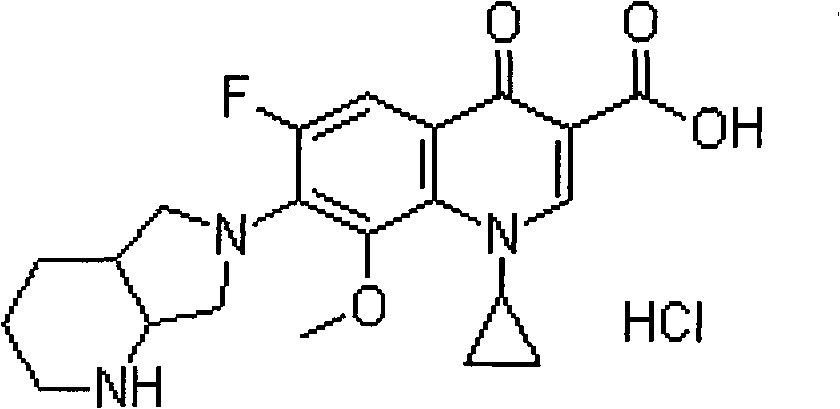
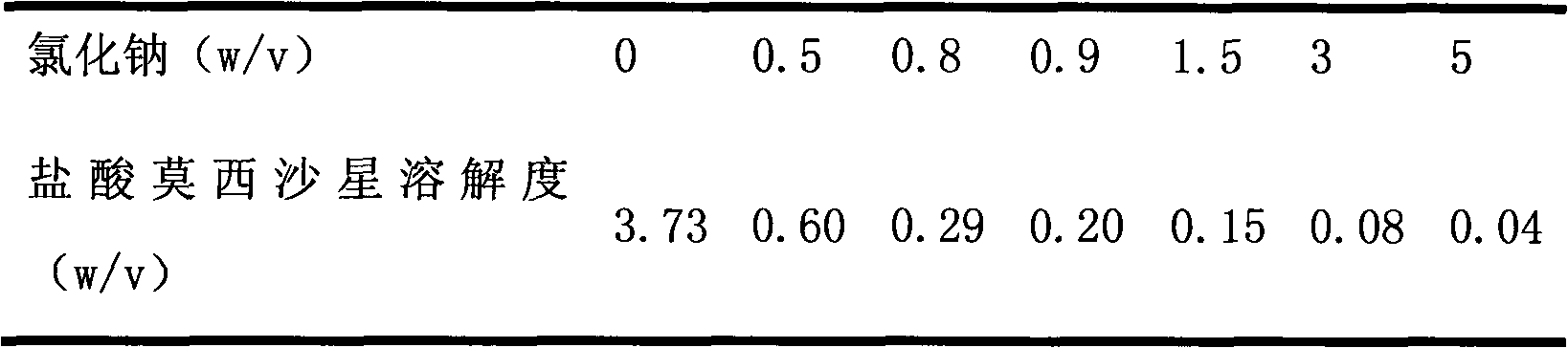
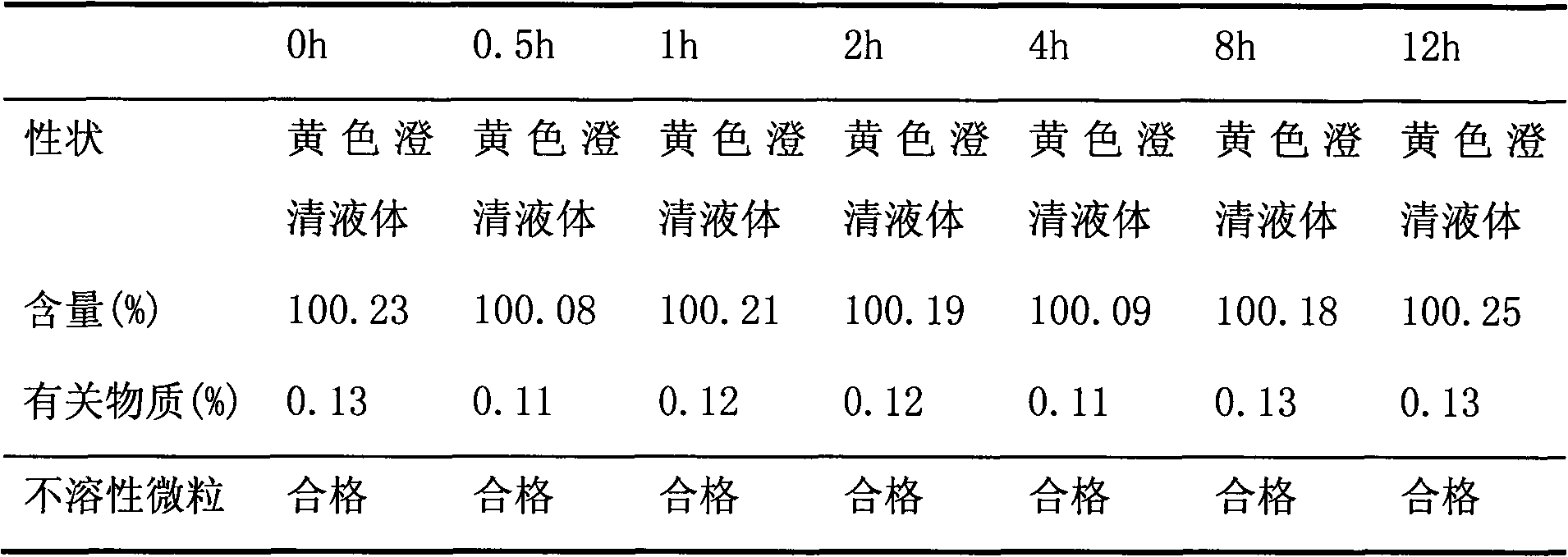
New moxifloxacin hydrochloride injection
A technology of moxifloxacin hydrochloride and injection, applied in the field of medicine, can solve the problems of poor operability, unqualified clarity, aggravated condensation reaction and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1. Preparation of Moxifloxacin Hydrochloride Injection 1
[0030] Moxifloxacin Hydrochloride Injection (20ml: 400mg)
[0031] prescription:
[0032] Moxifloxacin hydrochloride: 0.4Kg (based on moxifloxacin)
[0033] Water for injection: 20L
[0034] Total preparation: 1000
[0035] Preparation Process:
[0036] (1) Wash and sterilize the ampoules required for production;
[0037] (2) 80% of the water for injection is kept at 50°C, then add moxifloxacin hydrochloride and stir to dissolve;
[0038] (3) Adjust the pH to 5.0 with 0.5 mol / l sodium hydroxide solution, add water for injection to the full amount, and stir evenly;
[0039] (4) Add 0.1% activated carbon for needles, stir for 15 minutes, filter and remove carbon;
[0040] (5) The medicinal solution is finely filtered through 0.45μm and 0.22μm membrane filters;
[0041] (6) Determine the intermediates and potting after passing the inspection;
[0042] (7) Steam sterilization at 121 degrees for 15 minutes;
[0043] (8) Leak de...
Embodiment 2
[0064] Example 2. Preparation of Moxifloxacin Hydrochloride Injection 2
[0065] Moxifloxacin Hydrochloride Injection (10ml: 200mg)
[0066] prescription:
[0067] Moxifloxacin hydrochloride: 0.2Kg (based on moxifloxacin)
[0068] Water for injection: 10L
[0069] Total preparation: 1000
[0070] Preparation Process:
[0071] (1) Clean and sterile the vials and rubber stoppers required for production;
[0072] (2) 80% of the water for injection is kept at 50°C, then add moxifloxacin hydrochloride and stir to dissolve;
[0073] (3) Adjust the pH to 5.5 with 0.5 mol / l sodium hydroxide solution, add water for injection to the full amount, and stir evenly;
[0074] (4) Add activated carbon for needles with a solution amount of 0.05%, stir for 20 minutes, filter and remove carbon;
[0075] (5) The medicinal solution is finely filtered through 0.45μm and 0.22μm membrane filters;
[0076] (6) Determine the intermediates and potting after passing the inspection;
[0077] (7) Steam sterilization at 115 ...
Embodiment 3
[0086] Example 3. Preparation of Moxifloxacin Hydrochloride Injection 3
[0087] Moxifloxacin Hydrochloride Injection (20ml: 400mg)
[0088] prescription:
[0089] Moxifloxacin Hydrochloride: 400g (based on Moxifloxacin)
[0090] Calcium edetate sodium: 50g
[0091] Water for injection: 20L
[0092] Total preparation: 1000
[0093] Preparation Process:
[0094] (1) Clean and sterile the vials and rubber stoppers required for production;
[0095] (2) 80% of the water for injection is kept at 50°C, and the raw and auxiliary materials are added and stirred to dissolve;
[0096] (3) Adjust the pH to 5.0 with 0.5 mol / l sodium hydroxide solution, add water for injection to the full amount, and stir evenly;
[0097] (4) Add activated carbon for needles with a dosage of 0.05%, stir for 15 minutes, filter and remove carbon;
[0098] (5) The medicinal solution is finely filtered through 0.45μm and 0.22μm membrane filters;
[0099] (6) The liquid medicine is sterilized through a 0.22μm filter membrane in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com