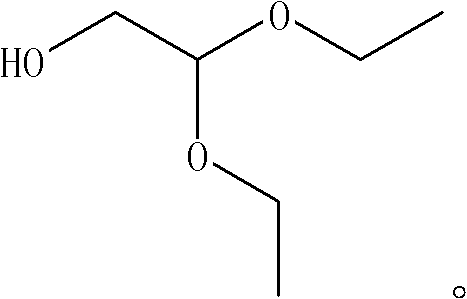
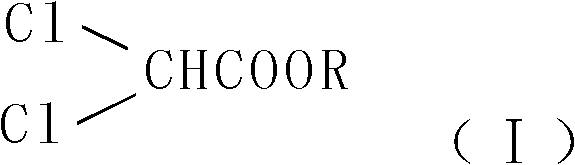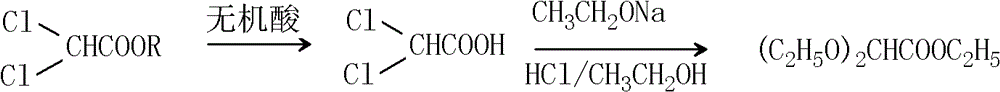Synthesis method of 2, 2-ethoxyethanol
A technique for the synthesis of diethoxyethanol, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of side reactions, low product purity and yield, and achieve sufficient reaction and product yield. High efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Put 400g of methyl dichloroacetate, 400ml of water and 40g of hydrochloric acid with a concentration of 36.5% into a 1000ml four-neck flask, raise the temperature to 80°C, and perform a hydrolysis reaction for 2 hours. After about 80g of water and methanol, use vacuum distillation to control the temperature below 100°C, and distill the water in the reaction system. , Collect the distilled fractions to obtain dichloroacetic acid.
[0036] Put 75g of dichloroacetic acid, 861g of 17.4% sodium ethoxide and 230g of absolute ethanol into a 1000ml four-neck flask, heat up to 80°C for reflux, and react for 4.5 hours. After the reaction, cool down to 0°C and start to drop 37 % Hydrochloric acid ethanol solution 127.5g, add dropwise, control the temperature below 10°C, after the dropwise addition is completed, heat up to 20°C for reaction, after the reaction, then cool down to 0°C, adjust the pH value to 6.8 with sodium ethylate, the whole During the pH adjustment process, contr...
Embodiment 2
[0039] Put 400g of methyl dichloroacetate, 400ml of water and 120g of 30.0% hydrochloric acid into a 1000ml four-neck flask, heat up to 80°C, and perform hydrolysis reaction for 4 hours. After about 100g of water and methanol, switch to vacuum distillation to control the temperature below 100°C, and distill the water in the reaction system. The distilled fractions are dichloroacetic acid.
[0040] Put 75g of the prepared dichloroacetic acid, 600g of 15% sodium ethoxide and 250g of absolute ethanol into a 1000ml four-necked bottle, raise the temperature to 76°C-80°C, and react for 6 hours. After the reaction, cool down to 0°C and start dripping Add 90g of hydrochloric acid ethanol solution with a concentration of 40%, and control the temperature below 10°C. After the dropwise addition, raise the temperature to 20°C for reaction. During the process, the temperature was controlled not to exceed 10°C. After adjustment, the stirring was continued for 1 hour, and the sodium chlorid...
Embodiment 3
[0043] Put 400g of ethyl dichloroacetate, 400ml of water and 50g of hydrochloric acid with a concentration of 35.0% into a 1000ml four-necked flask, heat up to 80°C, and perform a hydrolysis reaction for 3 hours. After the reaction, distill under normal pressure to remove the residual dichloroacetic acid After about 110g of ethyl ester, water and ethanol, switch to vacuum distillation, control the temperature to less than 100°C, and distill out the water in the reaction system. When the temperature is above ℃, collect the distilled fractions to obtain dichloroacetic acid.
[0044] Put 75g of the prepared dichloroacetic acid, 870g of 18% sodium ethoxide and 230g of absolute ethanol into a 1000ml four-necked bottle, raise the temperature to 70°C to 75°C, and react for 6 hours. After the reaction, cool down to 0°C and start dripping Add 140g of hydrochloric acid ethanol solution with a concentration of 30%, and control the temperature below 10°C. After the dropwise addition, rais...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com