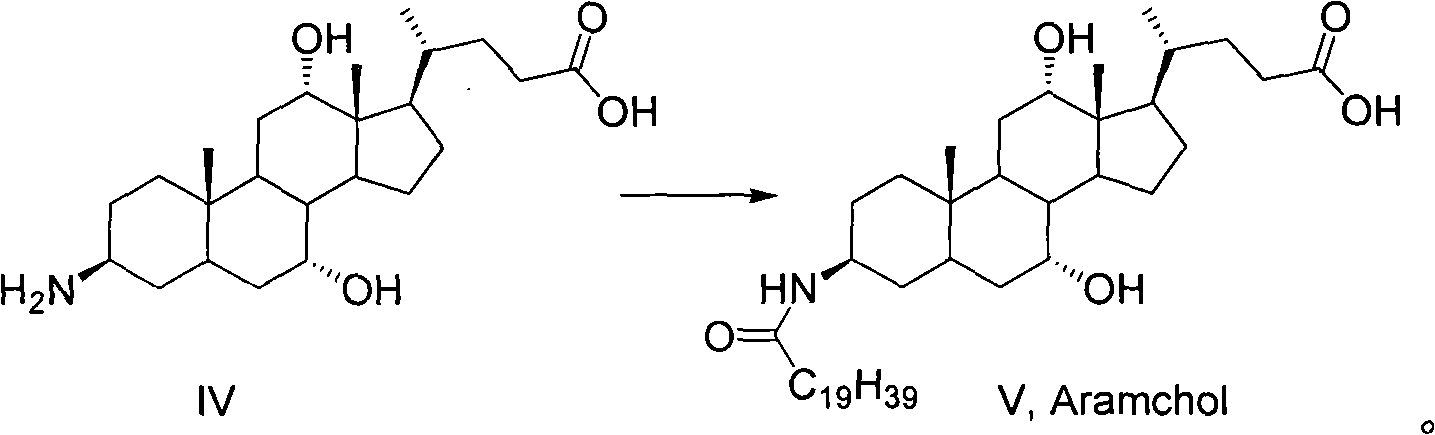
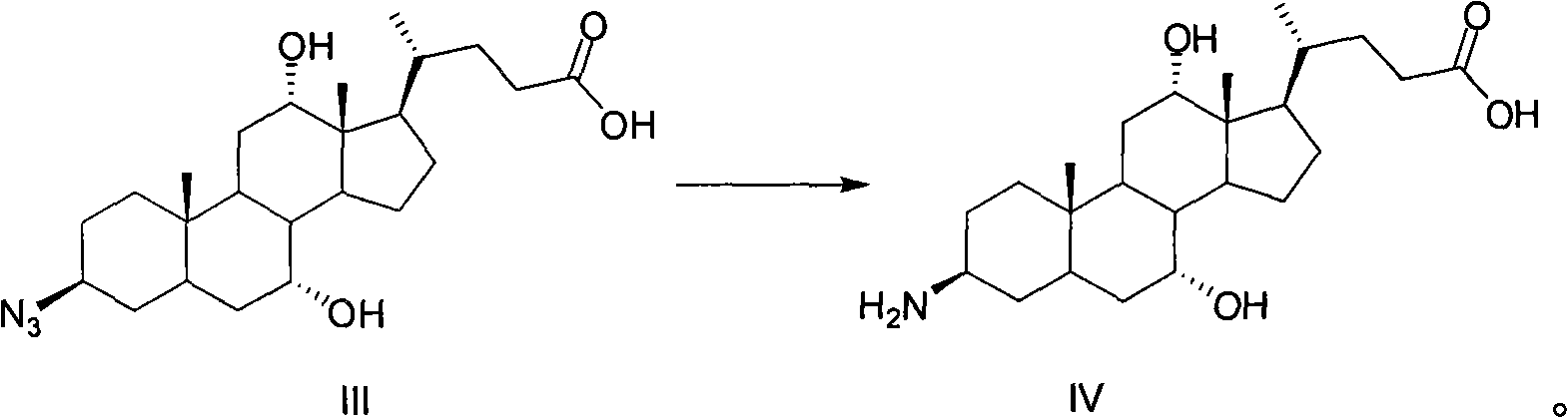
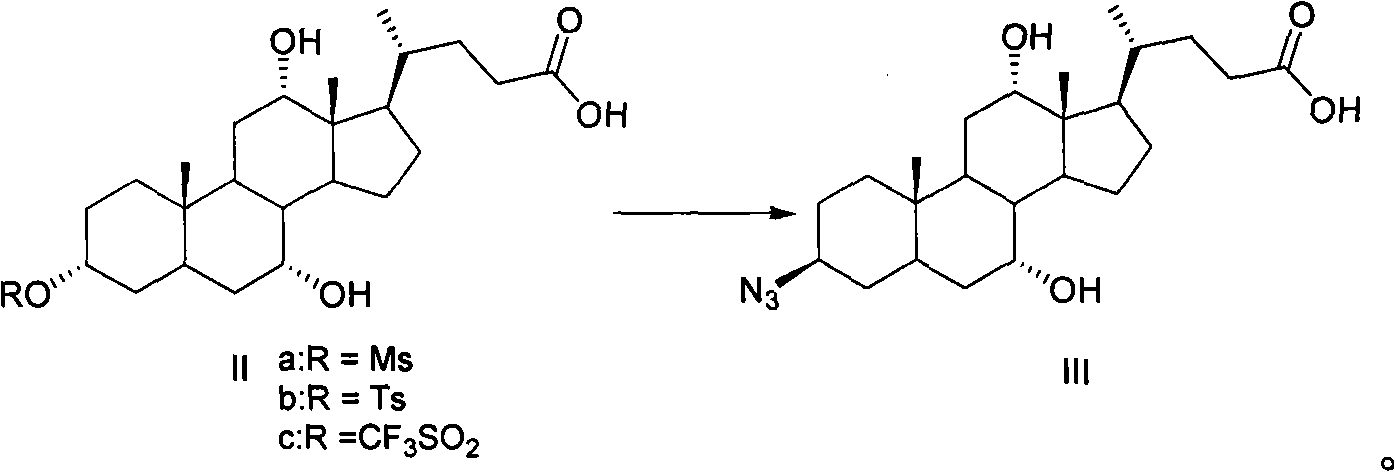
Preparation method of 3-beta-peanut amide-7alpha, 12alpha, 5beta-cholane-24-carboxylic acid
A technology of arachidonic amide group and arachidonic acid chloride is applied in the production of bulk chemicals, steroids, organic chemistry, etc., which can solve the problems of high consumption of raw materials and reagents, increase of synthesis cost, and impact on production efficiency, and achieves reduction of raw material consumption, The effect of short preparation cycle and improved production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of 3α-methanesulfonyloxy-7α, 12α-dihydroxy-5β-cholane-24-carboxylic acid (compound II-a)
[0028]
[0029] Dissolve 30 g (73.5 mmol) of cholic acid in 100 ml of pyridine, cool to 0°C, add 6.8 ml of methanesulfonyl chloride (88.2 mmol), and continue stirring at 0-10°C for 2 hours. Pour the reaction mixture into a mixture of 100 ml of ethyl acetate and 100 ml of water, stir, let stand, separate the organic layer, wash the water layer once with 100 ml of ethyl acetate, combine the organic phases, and wash with 50 ml of saturated brine , dried, and concentrated under reduced pressure to obtain white foamy solid II-a, which can be directly used in the next reaction.
Embodiment 2
[0030] Example 2: Preparation of 3α-methanesulfonyloxy-7α, 12α-dihydroxy-5β-cholane-24-carboxylic acid (compound II-a)
[0031] 30 g (73.5 mmol) of cholic acid was dissolved in 100 ml of pyridine and 100 ml of dichloromethane, cooled to 0°C, 6.8 ml of methanesulfonyl chloride (88.2 mmol) was added, and stirring was continued at 0-10°C for 2 hours. Pour the reaction mixture into a mixture of 100 ml of ethyl acetate and 100 ml of water, stir, let stand, separate the organic layer, wash the water layer once with 100 ml of ethyl acetate, combine the organic phases, and wash with 50 ml of saturated brine , dried, and concentrated under reduced pressure to obtain white foamy solid II-a, which can be directly used in the next reaction.
Embodiment 3
[0032] Example 3: Preparation of 3α-p-toluenesulfonyloxy-7α, 12α-dihydroxy-5β-cholane-24-carboxylic acid (compound II-b)
[0033]
[0034] Dissolve 30 g (73.5 mmol) of cholic acid in 100 ml of pyridine, cool to 0°C, add 16.8 g of p-toluenesulfonyl chloride (88.2 mmol), and continue stirring at 0-10°C for 2 hours. Pour the reaction mixture into a mixture of 100 ml of ethyl acetate and 100 ml of water, stir, let stand, separate the organic layer, wash the water layer once with 100 ml of ethyl acetate, combine the organic phases, and wash with 50 ml of saturated brine , dried, and concentrated under reduced pressure to obtain white foamy solid II-b, which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com