Ionic liquid segmented copolymer with imidazole-contained main chain and preparation method thereof
A technology of imidazole ionic liquid and block copolymer, applied in the field of ionic liquid block copolymer and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The steps of the preparation method of the ionic liquid block copolymer that main chain contains imidazole are as follows:
[0022] 1) in N 2 Under protection, mix imidazole and methyl acrylate at a molar ratio of 1:1.2, add 10 times the molar amount of the total amount of anhydrous methanol, under stirring, reflux reaction for 24h, rotary evaporation to remove methanol solvent and unreacted acrylic acid Methyl ester, wash off residual unreacted methyl acrylate with n-hexane to generate 1-(3-methoxy-3-oxypropyl) imidazole; the reaction equation is as follows:
[0023]
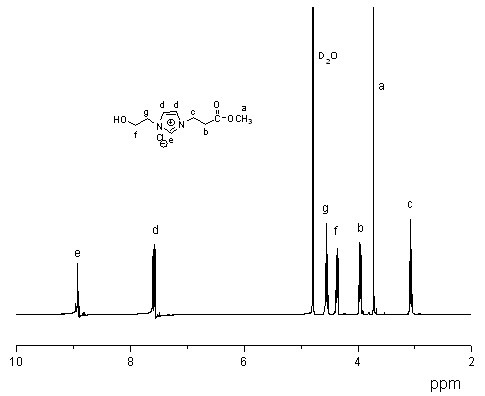
[0024] The NMR characterization of 1-(3-methoxy-3-oxypropyl)imidazole is as follows:
[0025]
[0026] 1 H-NMR (400MHz, δ, ppm; solvent is D 2 O)
[0027] a (δ=3.63 H3) b (δ=2.73 H2) c (δ=4.21 H2) d (δ=6.88 H1)
[0028] e (δ=6.98 H1) f (δ=7.45 H1)
[0029] Among them, a, b, c, d, e, and f represent the positions marked with H, respectively.
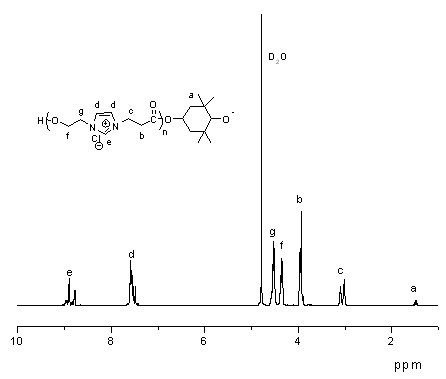
[0030] 2) at N 2 Under protection, 1-(3-methoxy-...
Embodiment 1
[0054] Equipped with magnetic stirring, reflux condenser, N 2 Add 200mL (10 times the molar amount) of anhydrous methanol to the 250mL three-necked flask of the catheter, N 2 Under protection, mix 14g imidazole with 21.244g methyl acrylate (1:1.2 molar ratio), reflux reaction for 24h, remove the methanol solvent and unreacted methyl acrylate by rotary evaporation at room temperature, and use soluble methyl acrylate insoluble reaction product Wash away the residual unreacted methyl acrylate with n-hexane to obtain a colorless and transparent liquid, namely 1-(3-methoxy-3-oxypropyl)imidazole;
[0055] in N 2 Under protection, mix 14.772g of 1-(3-methoxy-3-oxopropyl)imidazole with 7.7mL of 2-chloroethanol (1:1.2 molar ratio), add a total of 200mL (10 times molar amount) Toluene, under stirring, reacted at 80°C for 48h, and the liquid product precipitated. Dissolve in water first, then wash and extract unreacted 2-chloroethanol with chloroform, and dry in vacuum to obtain light...
Embodiment 2
[0062] Equipped with magnetic stirring, reflux condenser, N 2 Add 300mL (10 times the molar amount) of anhydrous methanol to the 250mL three-necked flask of the catheter, N 2 Under protection, 22.143g imidazole and 33.6g methyl acrylate were mixed (1:1.2 molar ratio), refluxed for 24h, and the methanol solvent and unreacted methyl acrylate were removed by rotary evaporation at room temperature. Wash away the residual unreacted methyl acrylate with n-hexane to obtain a colorless and transparent liquid, namely 1-(3-methoxy-3-oxypropyl)imidazole;
[0063] in N 2 Under protection, mix 12.640g of 1-(3-methoxy-3-oxopropyl)imidazole with 6.6mL of 2-chloroethanol (1:1.2 molar ratio), add a total of 200mL (10 times molar amount) Toluene, under stirring, reacted at 80°C for 48h, and the liquid product precipitated. Dissolve in water first, then wash and extract unreacted 2-chloroethanol with chloroform, and dry in vacuum to obtain light yellow transparent chlorinated 1-(3-methoxy-3-o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com