Fluorescent silver cluster, and preparation method and application thereof
A fluorescence and cluster technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of poor controllability, high cost, complicated process, etc., and achieve excellent stability, high sensitivity and specificity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of water-soluble silver clusters:
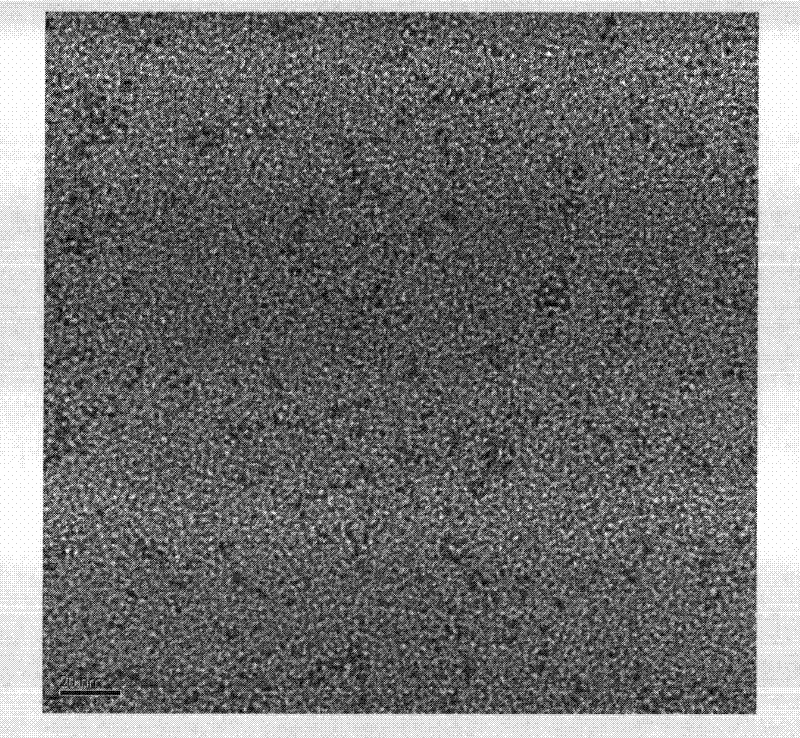
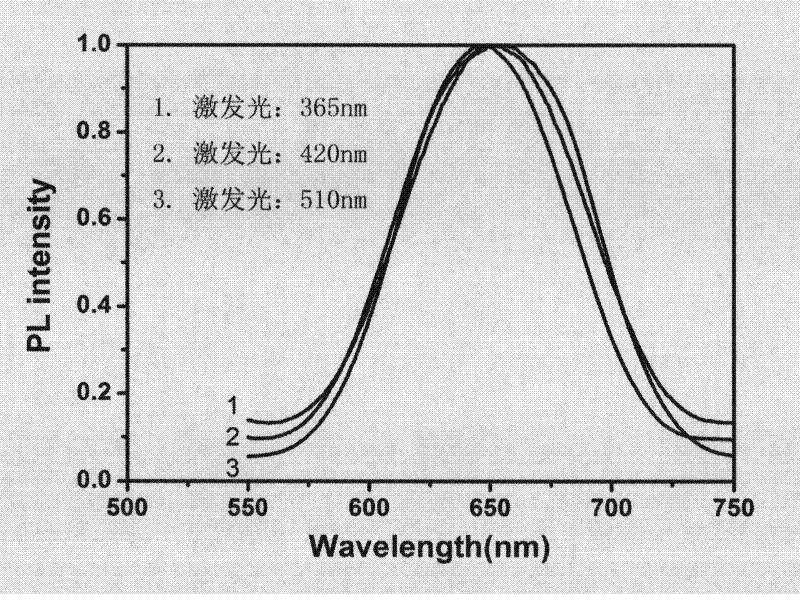
[0044] Add 20.63mg of lipoic acid (Hla) and 10ml of deionized water into a round bottom flask, stir quickly, most of the lipoic acid will not dissolve; then slowly drop 100ul of 1mol / L NaOH aqueous solution, the hydrogen ion of the carboxyl group of lipoic acid It is neutralized by NaOH and dissolved in water; then add 100ul of AgNO3 solution with a concentration of 25mM. After stirring for five minutes, slowly add 100ul of newly prepared NaBH4 solution with a concentration of 300mM. Observe that the solution will gradually turn brown, and then use tin foil. Keep away from light, overnight at room temperature; take it out the next day, the solution turns light yellow, that is, the required fluorescent silver cluster particles are generated. Observed by transmission electron microscope, the cluster size is found to be about 1nm (see figure 1 ), its optical properties such as figure 2 As shown, the emission wavelength...
Embodiment 2
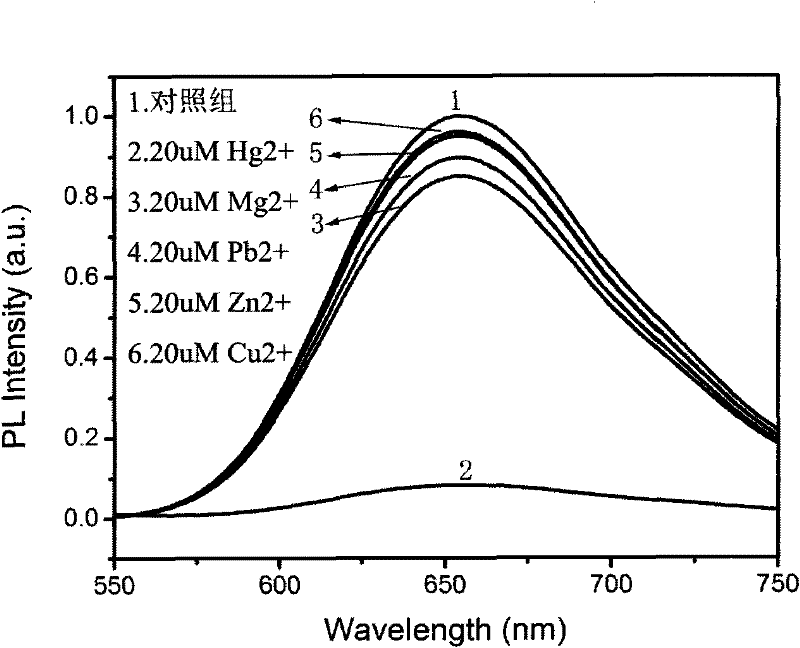
[0047] Example 2 Fluorescence experiment on the selectivity of silver cluster particles to various ions:
[0048] Take the same volume of silver cluster solution and use metal ion salt (Hg 2+ , Mg 2+ , Pb 2+ , Zn 2+ , Cu 2+ ) Make the same solution concentration, use a pipette to take a small volume of the solution from each of these metal ion salts, and add it to the prepared silver cluster aqueous solution. The final concentration of various ions is 20uM. Add the above ions The fluorescence of the latter solution was quickly measured with a fluorescence spectrophotometer, and the fluorescence spectrum was measured at 420nm. The results are shown in the attached file. image 3 .
Embodiment 3
[0049] Example 3 Fluorescence response experiment of silver cluster nanoparticles to various concentrations of mercury ions:
[0050] Take the same volume of silver cluster solution, and mix the mercury salt into solutions of different concentrations according to the gradient, then use a pipette to take a small volume of the solution from each of these mercury ion salts, and add them to the prepared silver cluster aqueous solution , Measure the fluorescence of the above-mentioned solution after adding ions quickly with a fluorescence spectrophotometer, select excitation at 420nm, and measure the fluorescence spectrum. The results are shown in the attached file. Figure 4 .
[0051] In summary, the present invention utilizes the solubility of lipoic acid under alkaline conditions and the easy coordination of sulfur elements with silver ions, and proposes a water-soluble fluorescent silver cluster and its preparation method. The size of the fluorescent silver cluster is very high. S...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com