Method for removing organochlorine from hydrocarbon oil
A technology for organochlorine and hydrocarbon oils, applied in chemical dehydration/demulsification, electric/magnetic dehydration/demulsification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
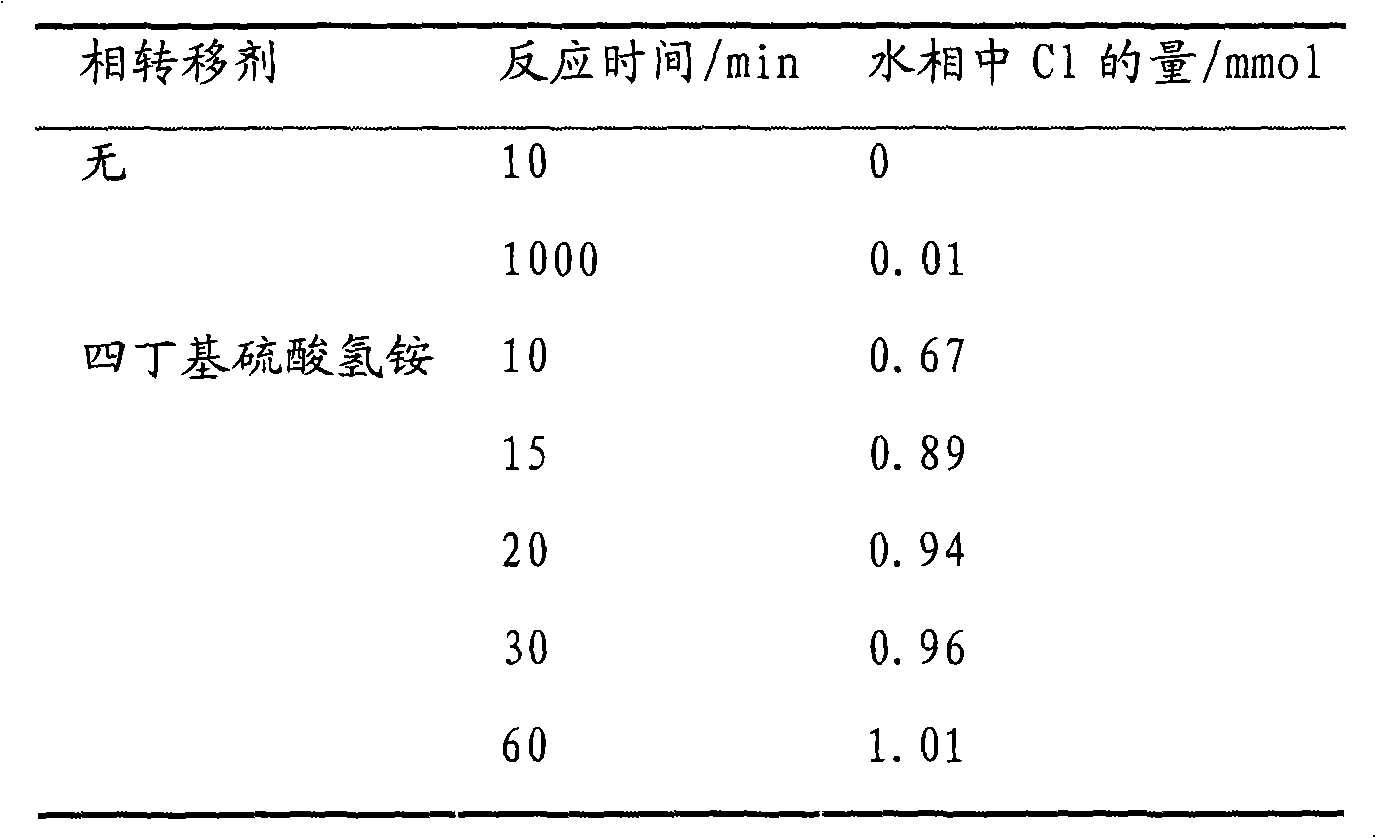
[0028] The experiment uses dichloromethane model oil to investigate the hydrolysis of dichloromethane, that is, the situation where organic chlorine is replaced by hydroxyl under the condition that there are alkalis and quaternary ammonium compounds in the water phase. The quaternary ammonium compound is tetrabutylammonium hydrogensulfate. In the experiment, 10ml of dichloromethane was taken, 5g of sodium hydroxide, 0.34g (1mmol) of tetrabutylammonium hydrogensulfate were dissolved in 5ml of water, stirred at room temperature, and analyzed in the water phase. The concentration of chloride ions was calculated, and the amount of chlorine replaced was calculated. The experimental results are shown in Table 1. It can be seen from the experimental results that without adding a phase transfer agent, only 0.01mmol of Cl hydrolyzes into the water phase after 1000min, adding 1mmol of the phase transfer agent NBu 4 HSO 4 , stirred at room temperature for 60min, an equimolar amount of C...
Embodiment 2
[0032] The density of crude oil used is 0.9331g / cm 3 , the salt content is 34mgNaCl / L, the total chlorine content is 44mgNaCl / L, and the difference between total chlorine and salt content is the organic chlorine content of 10mgNaCl / L.
[0033] The demulsifier used is alkyl phenolic resin polyoxyethylene polyoxypropylene ether, the brand name is F 3111, and the dosage is 50 μg / ; the basic compound is made into a solution in proportion, diethanolamine: ethylenediamine: NH 3 ·H 2 O: water=30:5:35:30, the amount of basic compound solution is 200 μg / g; the amount of tetrabutylammonium bisulfate is 50 μg / g.
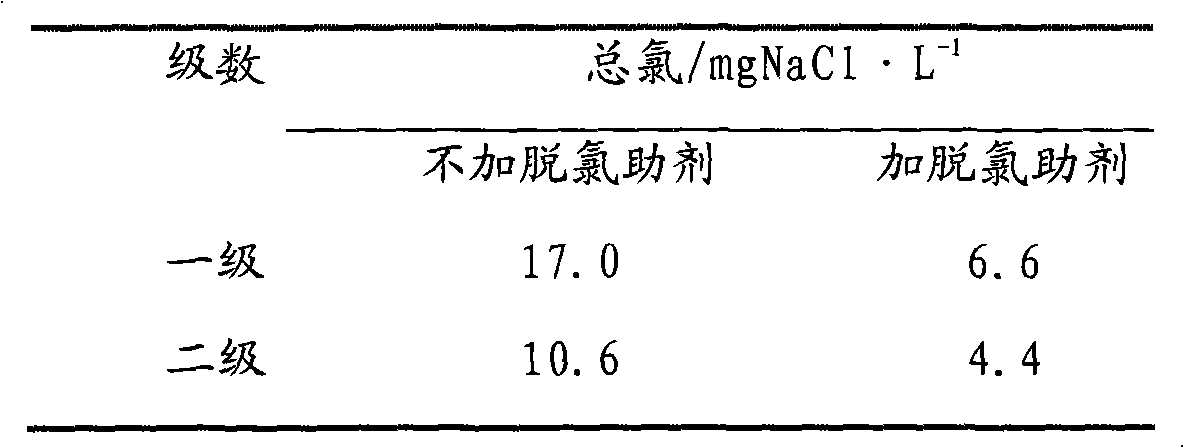
[0034] Mix the demulsifier, alkaline compound solution and tetrabutylammonium bisulfate with water injection, the water injection rate is 10%, mix the aqueous solution with the crude oil preheated to 90-100°C on a commercially available juicer for 20 seconds, using DP-2C The demulsifier selection instrument is used for oil-water separation, the electric field strength is 300V...
Embodiment 3
[0039] Raw oil density 0.9506g / cm 3 , the salt content is 40mgNaCl / L, the total chlorine content is 114.35mgNaCl / L, and the organic chlorine content is 74.35mgNaCl / L.
[0040] The demulsifier used is polyethylene polyamine polyoxypropylene ether, the trade name is AE9901, and the dosage is 100 μg / g; the basic compound is made into a solution in proportion, triethanolamine: dimethylamine: NaOH: water = 20:5:20 : The amount of 55 basic compound solution is 500 μg / g; the amount of cetyltrimethylammonium hydroxide is 300 μg / g.
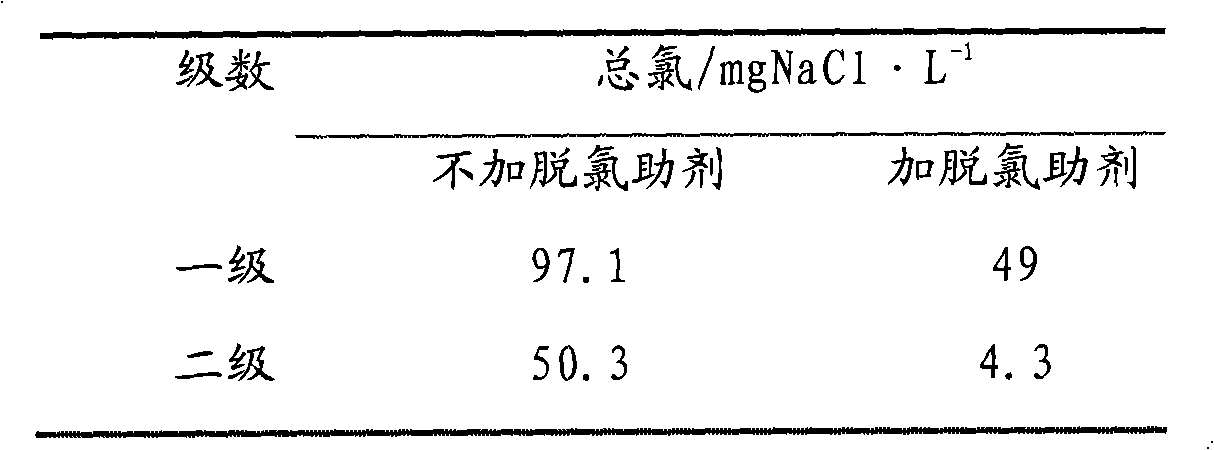
[0041] The desalination method is the same as in Example 2, and the experimental results are shown in Table 3. It can be seen from the experimental results that the total chlorine content after two-stage dechlorination is 50.3 mgNaCl / L without adding dechlorination aids, and the total chlorine content drops to 4.3 mgNaCl / L after adding dechlorination aids.
[0042] table 3
[0043]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com