Ifenprodil tartrate freeze-dried powder injection and preparation method thereof
A technology of ifenprodil tartrate and freeze-dried powder injection, which is applied in the field of ifenprodil tartrate freeze-dried powder injection and its preparation, which can solve the problems of easy oxidation, substance increase, high-heat instability, etc., to improve stability, Good stability and high quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
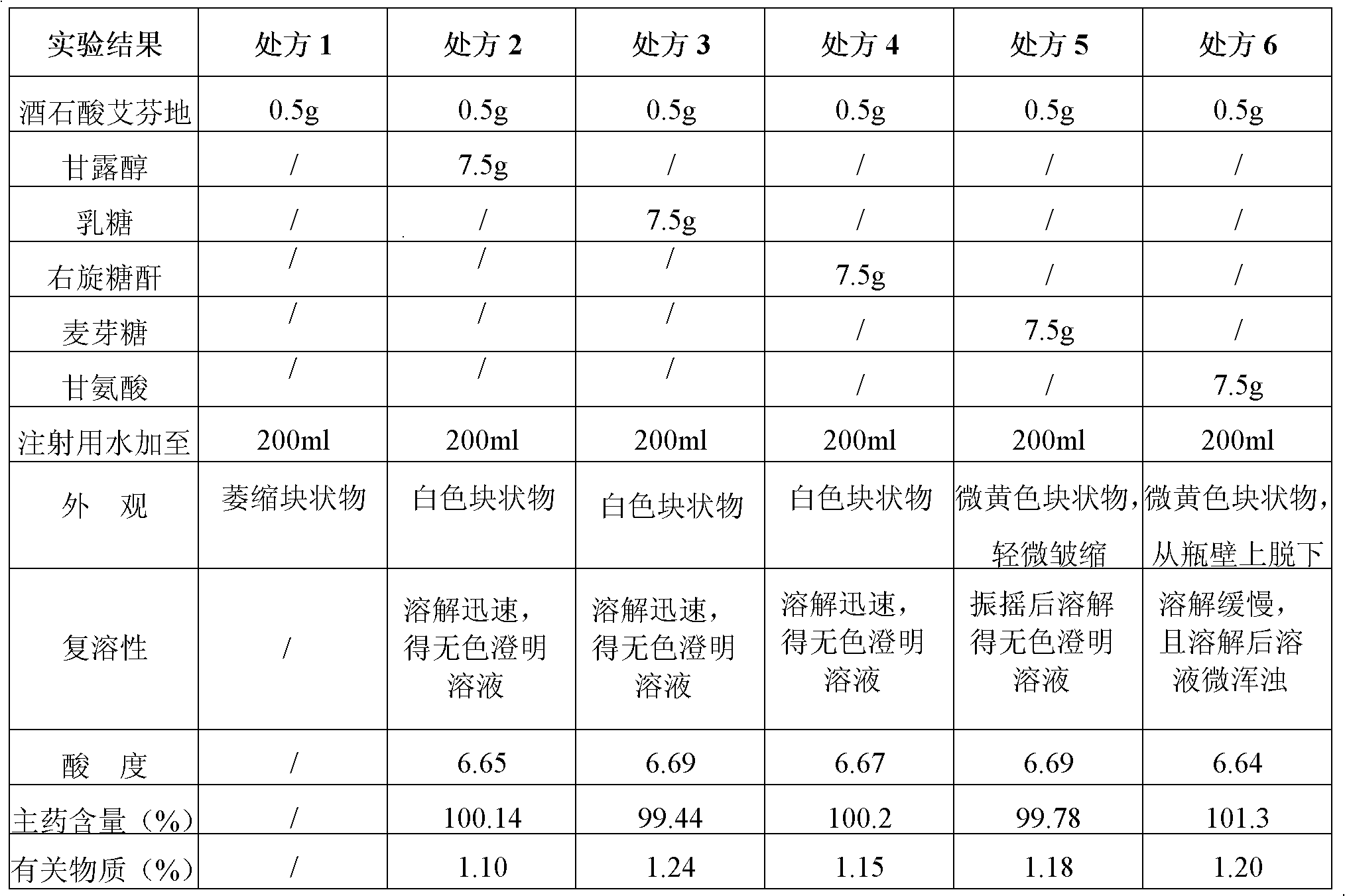
[0042] Experimental Example 1 Prescription Screening Experiment
[0043] Using mannitol, lactose, dextran, maltose, and glycine as excipients, weigh the main ingredients and excipients as shown in Table 1, and prepare freeze-dried powder injections according to the following preparation methods. , main drug content and related substances are the evaluation indicators, respectively investigate the formability of ifenprodil tartrate freeze-dried powder injection, the results are shown in Table 1:
[0044] The preparation method of freeze-dried powder injection is as follows:
[0045] ①Weigh the prescribed amount of ifenprodil tartrate and excipients, add 90% of the prescribed amount of water for injection to dissolve at 50°C, add 0.03% (g / ml) of activated carbon for needle adsorption for 30min, add water for injection to the full amount, filter to remove carbon;
[0046] ② Check the pH value and content of the filtrate, calculate the filling volume, filter the solution through...
experiment example 2
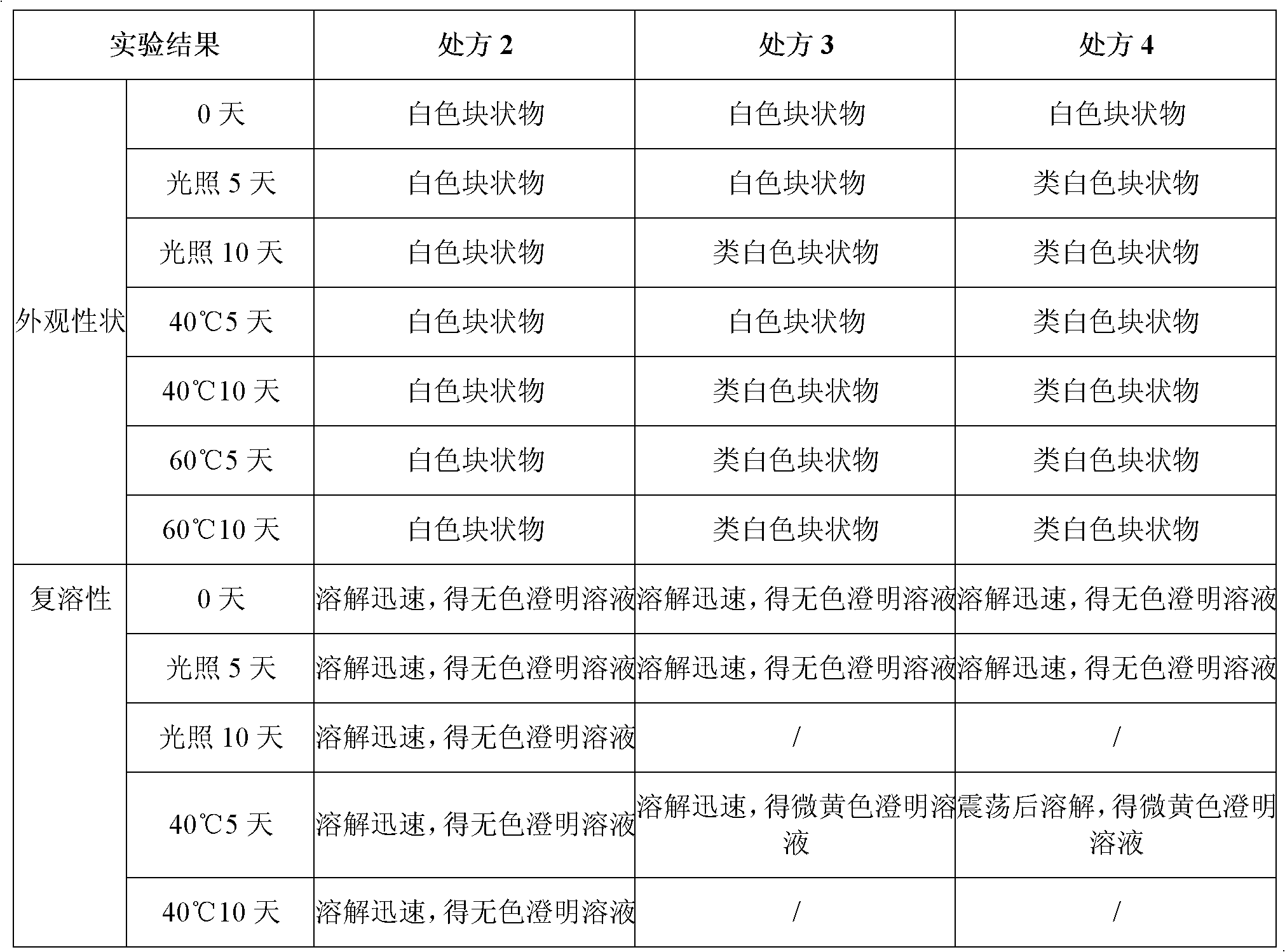
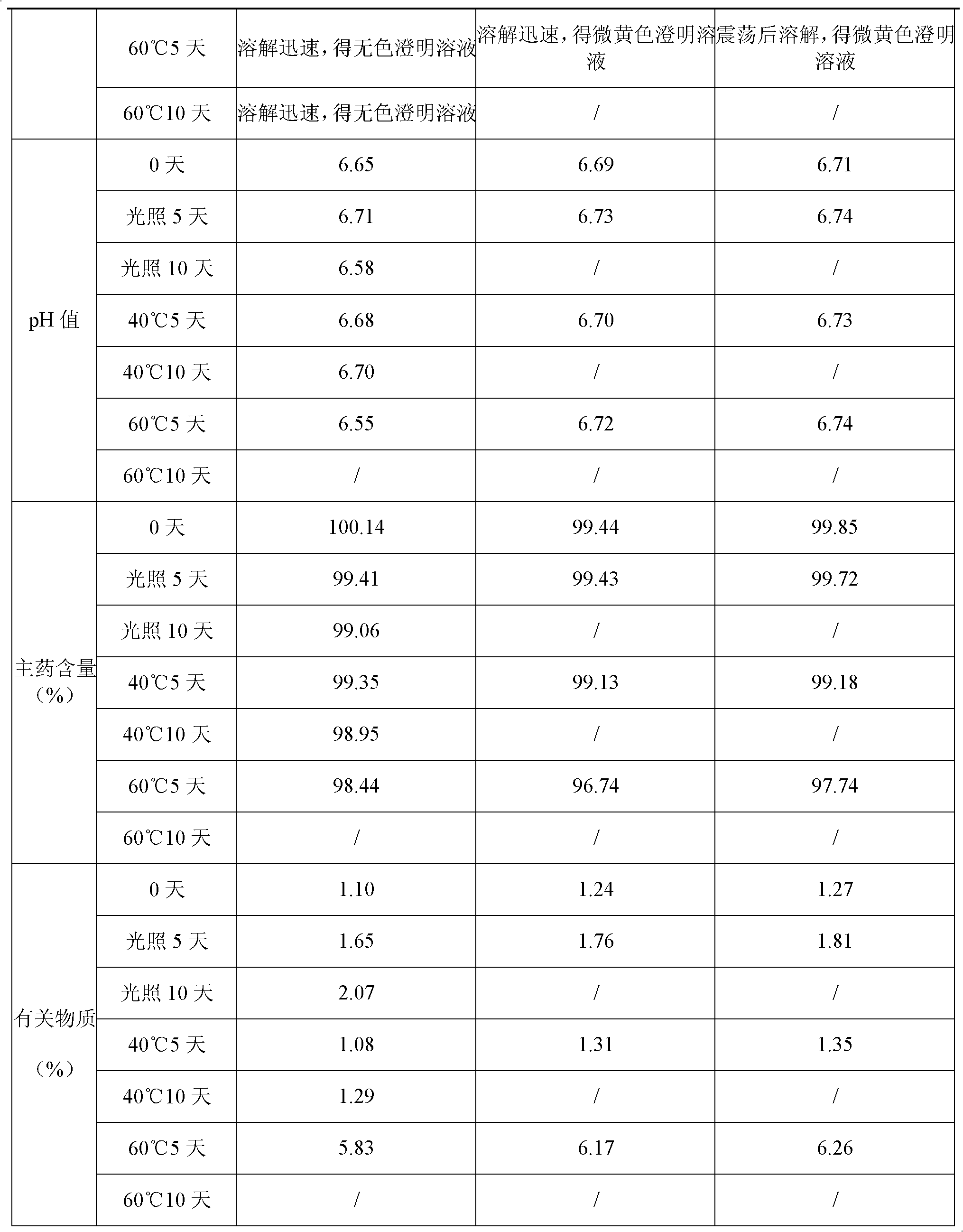
[0062] Experimental example 2 preparation process research
[0063] 1. Effect of activated carbon dosage and decarbonization method on main drug content and related substances
[0064] Activated carbon for needles is used in the preparation of injections to adsorb heat sources, impurities and pigments in raw and auxiliary materials to improve product quality; but if the amount is too large, it will adsorb the main drug to reduce the content of the main drug or introduce other impurities into the finished preparation. The decarbonization method in the liquid preparation process will have a certain impact on the related substances of this product, so the amount of activated carbon and the decarburization method are screened and studied with the content of the main drug and related substances as the assessment indicators.
[0065] Table 3 Effects of activated carbon dosage and decarbonization method on the main drug content and related substances
[0066]
[0067]
[0068]...
Embodiment 1
[0085]
[0086] ① Weigh the prescribed amount of mannitol and add about 90% water for injection, stir to dissolve it, add 0.05% (g / ml) activated carbon for needles into the solution, absorb for 30 minutes, filter and decarbonize;
[0087] ② Add the prescribed amount of ifenprodil tartrate raw material to the filtrate (temperature controlled at 50°C), stir to dissolve completely, cool down to room temperature, and add water for injection to the full amount;
[0088] ③ Check the pH value and content of the solution, calculate the filling volume, filter the solution through a 0.22 μm microporous membrane, and fill it;
[0089] ④ Freeze drying:
[0090] a. Turn on the cooling of the front box to reduce the temperature of the product to about -40°C and keep it for 2 to 3 hours;
[0091] b. Turn on the cooling of the rear box to reduce the temperature of the condenser to about -50°C;
[0092] c. Evacuate the vacuum of the freeze-drying box to below 10Pa;
[0093] d. One-time s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com