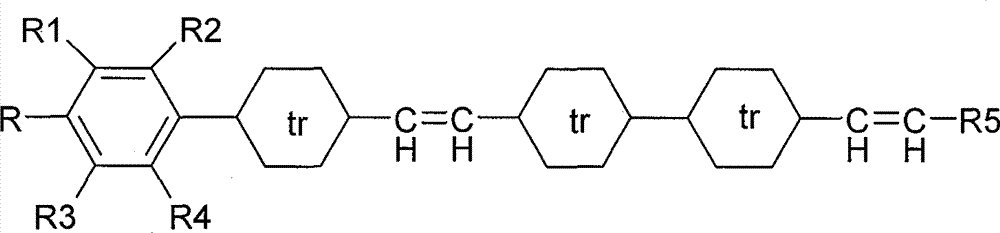
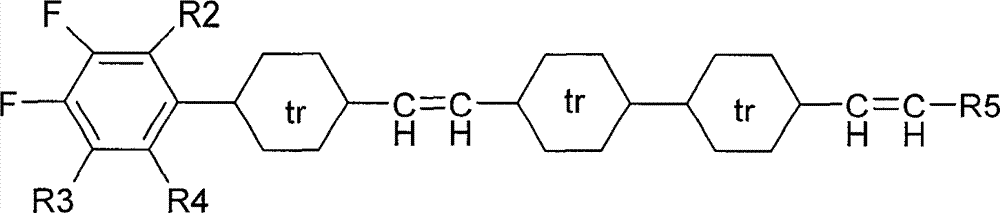
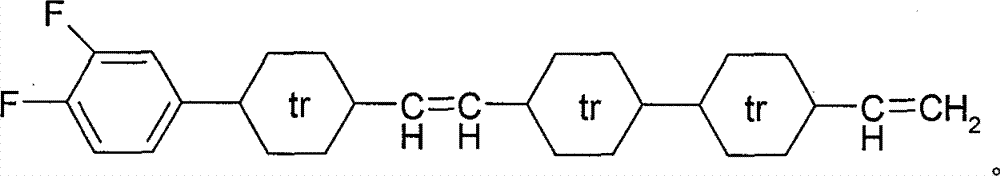
Novel tetracyclic diene liquid crystal compound and preparation method thereof
A technology of liquid crystal compound and tetracyclic diene, which is applied in the field of new tetracyclic diene liquid crystal compound and its preparation, can solve problems such as small dielectric anisotropy value, improve steepness and viewing angle, avoid side reactions, design reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052]Preparation of Example 14-(2-(4-(3,4-difluorophenyl)cyclohexyl)vinyl)-4'-ethylene bicyclohexane
[0053]
[0054] 3,4-difluorophenyl cyclohexyl ketone 80g, according to 3,4-difluorophenyl cyclohexyl ketone: chloromethyl ether phosphine salt: potassium tert-butoxide (mol: mol: mol) = 1: 1.2: 1.5 feeding . Put 300mL tetrahydrofuran solution containing chloromethyl ether phosphonium salt into a 1000mL four-neck flask, cool to below -10°C under nitrogen protection, maintain for 10min, then gradually add potassium tert-butoxide and keep stirring for 30min below 10°C. Add dropwise 3,4-difluorophenylcyclohexyl ketone in 300 mL tetrahydrofuran at below 10°C. After the addition is complete, let it rise to room temperature naturally and react for 4 hours. After the reaction was completed, an appropriate amount of saturated solution of sodium bicarbonate was added until no white precipitate was formed. Separate the liquid, dry the organic phase with anhydrous sodium sulfate ov...
Embodiment 2
[0087] Preparation of Example 24-(2-(4-p-tolylcyclohexyl)vinyl)-4'-ethylene bicyclohexane
[0088] In the process of preparing intermediate I, p-methylphenylcyclohexyl ketone was used as a raw material to react with phosphonium chloromethyl ether to prepare vinyl ether through Wittig reaction, and the rest of the process was the same as that of Example 1. 4-(2-(4-p-tolylcyclohexyl) vinyl)-4'-ethylene bicyclohexane can be obtained, and its structural formula is as follows:
[0089]
[0090] The synthesized compounds were characterized by MS and 1HMR methods to confirm their structures.
[0091]
[0092] MS: m / z: 726.70 (100.0%), 727.71 (58.4%), 728.71 (16.7%), 729.71 (3.0%)
[0093] MP: 190.3°C
[0094] 1 H NMR (CDCl 3 / TMS)δ H : 7.26~6.90(m, 4H, H 1 );5.80~5.73(m,1H,H 2 ); 5.34(t, 2H, H 3 , J=4.4Hz); 4.97~4.86(m, 2H, H 4 ); 2.42(t,1H,H 5 , J=4.9Hz); 2.39(s, 3H, H 9 ); 1.94~1.79(m, 12H, H 7 ); 1.53~1.42(m, 2H, H 5 ); 1.40~1.37(m, 3H, H 6 ); 1.25~1.02(m, 12H, H...
Embodiment 3
[0097] In the same way, use
[0098]
[0099] Used in the process of preparing intermediate I in replacing embodiment 2
[0100]
[0101] Other process is identical with embodiment 2, can finally make:
[0102]
[0103] MP: 199.1°C
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com