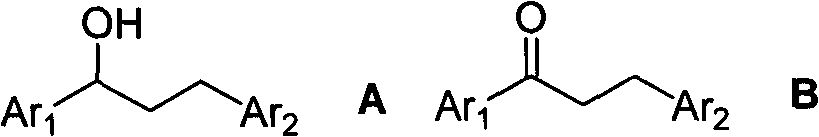Method for synthesizing 1,3-diphenyl-1-propanol compound
A synthesis method and diphenyl technology are applied in the field of cross-coupling reaction between secondary alcohol and primary alcohol, which can solve the problems of unsuitable obtainment of ligands, the need for special preparation, and the increase of post-processing costs, so as to reduce the difficulty of purification and post-processing. Reduced environmental impact and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Under nitrogen, ferrocene 0.1mmol, 18.6mg, Cs 2 CO 3 1.0mmol, 32.6mg, 1-phenyl-ethanol 10.0mmol, 1.22g and benzyl alcohol 15.0mmol, 1.62g were sequentially added to 6.7mL of anhydrous m-xylene; stirred at 125°C for 8h. Cool to NH 4 Neutralize the reaction solution with a saturated Cl solution, extract it with ethyl acetate, and concentrate the extract in vacuo until there is no ethyl acetate odor; purify it by 100-200 mesh forward silica gel column chromatography, n-hexane: ethyl acetate = 40:1 After elution, the product 1,3-diphenyl-1-propanol 1.55g, 7.3mmol, yield: 73% can be obtained.
Embodiment 2
[0039] Under nitrogen, ferrocene formaldehyde 0.5mmol, 107.0mg, NaOH 2.0mmol, 80.0mg, 1-phenyl-ethanol 10.0mmol, 1.22g and 4-methoxybenzyl alcohol 10.0mmol, 1.38g were added in sequence for 10.0 mL of anhydrous p-xylene; at 130 ° C, stirred for 24h. Cool to NH 4 The reaction solution was neutralized with a saturated Cl solution, extracted with ethyl acetate, and the extract was concentrated in vacuo until there was no ethyl acetate smell. Purified by 100-200 mesh forward silica gel column chromatography, eluting with n-hexane:ethyl acetate=30:1, the product 1-phenyl-3-(4'-methoxyphenyl)-1-propane can be obtained Alcohol 2.33g, 9.6mmol, yield: 96%.
Embodiment 3
[0041] Under nitrogen, ferrocenemethanol 1.0mmol, 216.1mg, KOH 1.5mmol, 84.2mg, 1-(4'-methylphenyl)-ethanol 10.0mmol, 1.36g and 4-chlorobenzyl alcohol 20.0mmol, 2.85 g, successively added to 5.0 mL of anhydrous o-xylene. At 135°C, stir for 12h; cool, add NH 4 Neutralize the reaction solution with a saturated Cl solution, extract it with ethyl acetate, and concentrate the extract in vacuo until there is no ethyl acetate odor; purify it by 100-200 mesh forward silica gel column chromatography, n-hexane: ethyl acetate = 38:1 After elution, the product 1-(4'-methylphenyl)-3-(4'-chlorophenyl)-1-propanol 2.11g, 8.1mmol, yield: 81% can be obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com