Magnesium hydroxide
A magnesium hydroxide, magnesium oxide technology, applied in magnesium hydroxide, conductors, cables, etc., can solve problems such as health risks, scarcity of minerals, and continuous demand
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The preparation of the polymeric compositions of the present invention may be accomplished by any suitable mixing method known in the art, which methods will be apparent to those skilled in the art. The method involves dry blending of the individual components or their precursors, and subsequent processing in a conventional manner.
[0067] For thermoplastic polymeric compositions, the processing can include melt mixing either directly in the extruder used to make articles from the composition, or premixed in a separate mixing device such as a Banbury mixer. Alternatively, the dry blend of the components can be directly injection molded without pre-melt mixing.
[0068] When the filler material produced according to the invention contains more than one component, it can be prepared by intimately mixing its components together. The filler material is then suitably dry blended with the polymer and any desired additional components prior to processing as described above. ...
Embodiment 1
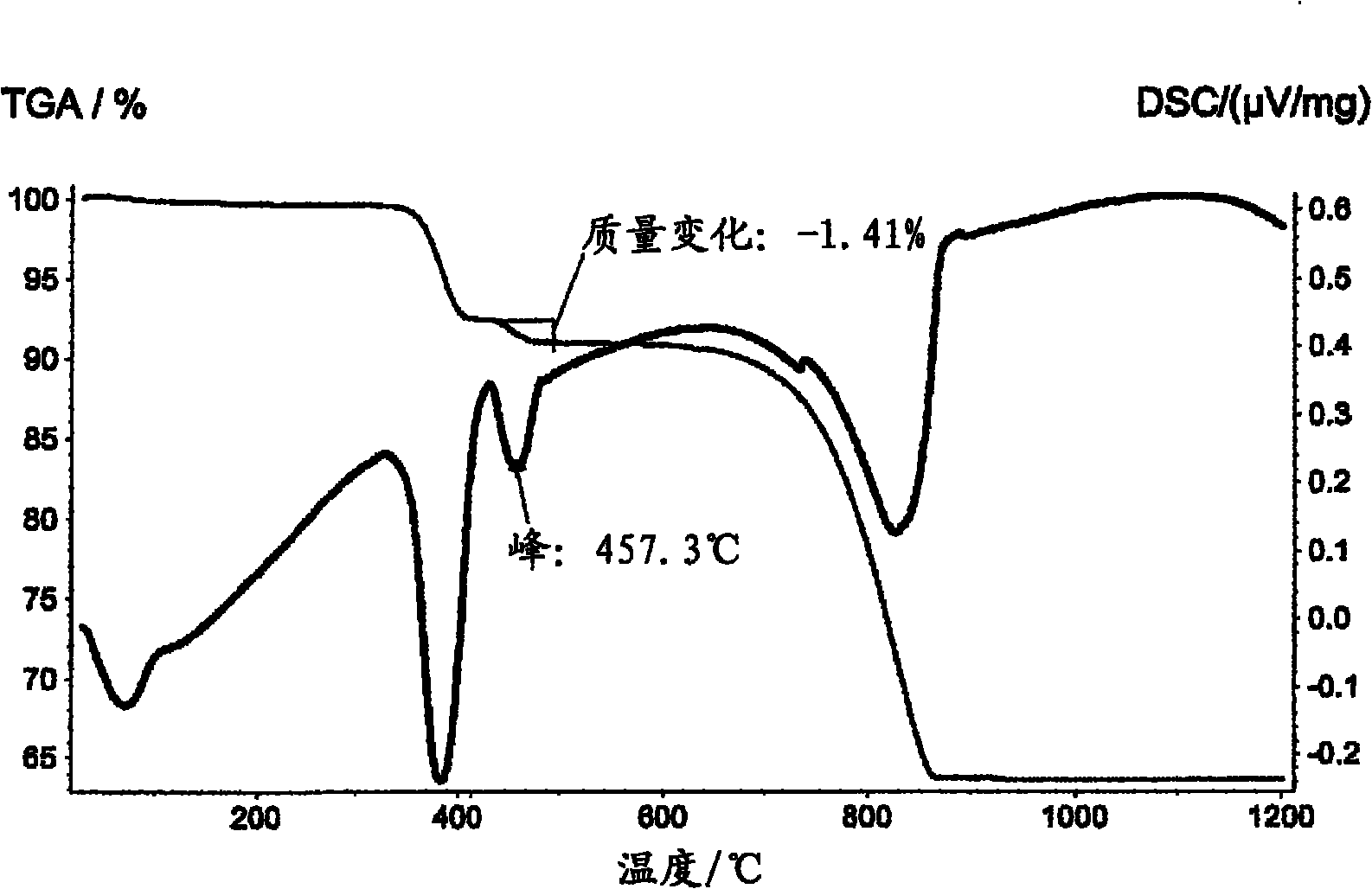
[0081] Samples of ground ore were loaded into refractory trays in a gas fired kiln manufactured by CGE, Skelmersdale, UK. The correct temperature for calcination is determined by DSC / TGA analysis of the minerals. For magnesite and dolomite samples, a 1 hour programmed period at about 850°C is appropriate.
[0082] 400 g of dry calcined feedstock for each sample was added to 4 liters of 50°C water in a stirred stainless steel vessel over 30 minutes and the temperature was maintained at 50°C by electrical heating. Afterwards, the suspension was screened to remove all particles larger than 53 μm. The screened suspension was allowed to cool and stand for 16 hours (overnight). The clear supernatant was then decanted and the remaining (thickened) suspension was vacuum filtered using standard laboratory Buchner filtration equipment. The obtained filter cake was dried at 80° C. to constant weight using a forced air oven. Post-drying milling was performed using a laboratory Fritsch...
Embodiment 2
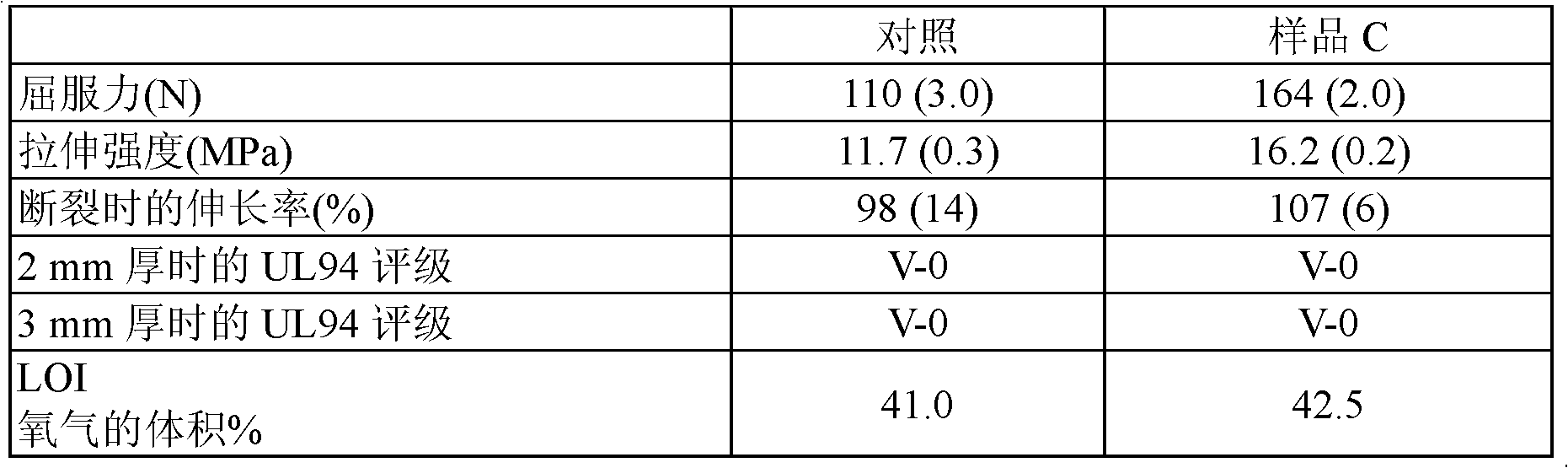
[0103]The flame retardant MDH filler (sample C) was prepared by calcining magnesium carbonate (95% pure by X-ray diffraction analysis) at 800°C followed by aging at 40°C followed by a simple settling step to provide a refined product, There are basically no particles larger than 20 μm in its particle size distribution. In Table 4 the physical properties of this filler are compared with a commercially available hydrotalcite mineral (Hydrofy G2.5, obtained from Nuova Sima).
[0104] Table 4
[0105]
Commercially available hydrotalcite
Sample C
[0106] Decomposed TGA loss*(%)
23.2
25.4
Surface area (m 2 / g)
7.0
22.4
CILAS d 50 ** (μm)
5.7
2.5
CILAS d 90 (μm)
24.6
7.9
CILAS d 99 (μm)
43.0
14.0
MgO(%)
56.3
64.6
CaO(%)
6.5
1.8
Fe 2 o 3 (ppm)
2560
2000
Mn(ppm)
161
100
[0107] As deta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com