Method for controllably preparing hydroxyl oxidize iron, iron sesquioxide and ferroferric oxide
A technology of iron oxyhydroxide and ferrous salt, which is applied in the direction of iron oxide/iron hydroxide, iron oxide, ferrous oxide, etc., to achieve the effects of cost reduction, low energy consumption, and green and safe reaction environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
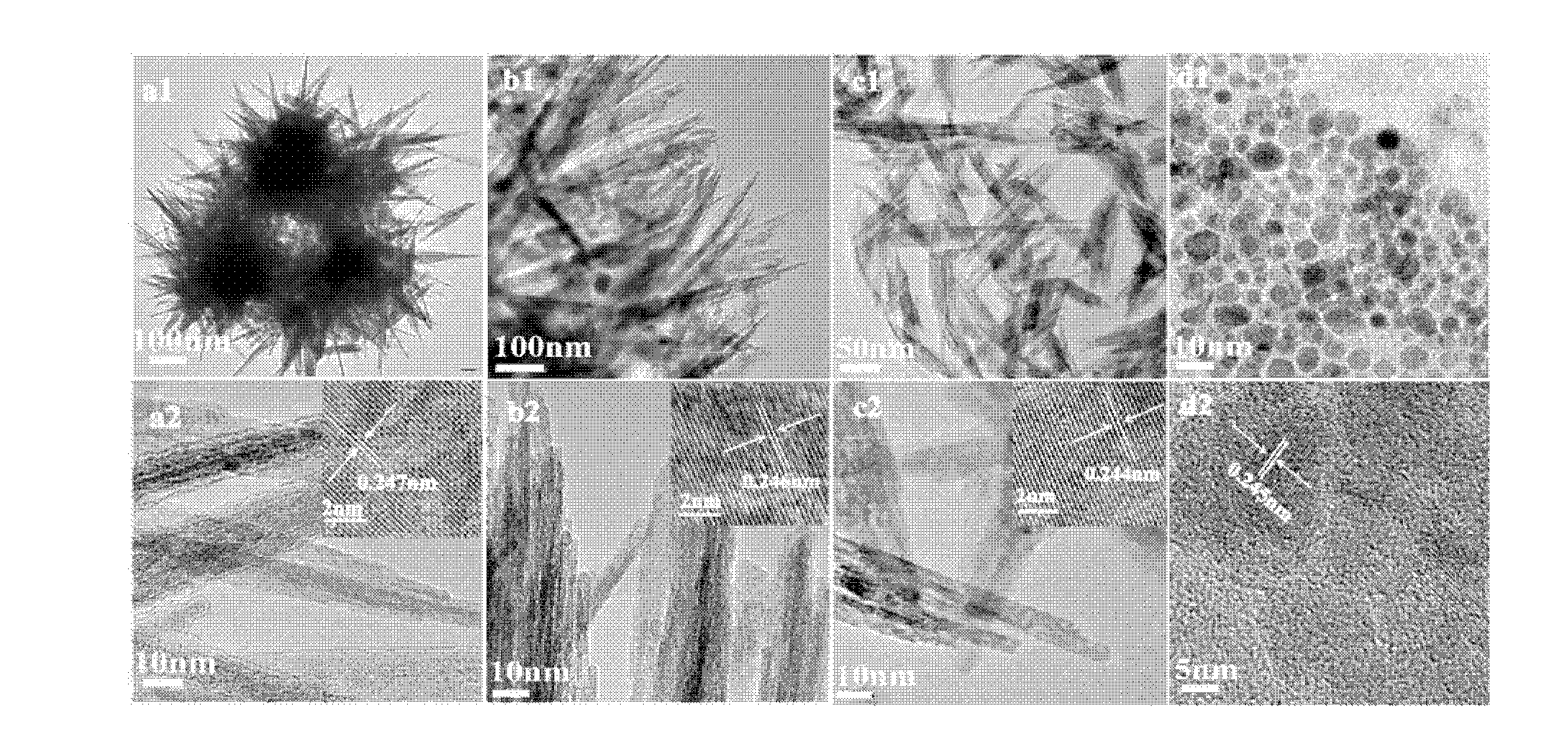
[0032] Embodiment 1: Preparation of sea urchin-shaped iron oxyhydroxide, ferric oxide, ferric oxide
[0033] Weigh 10g FeSO 4 ·7H 2 Dissolve O in 100mL deionized water, stir to dissolve completely, then add 10mL ethylene glycol to it, and continue stirring for 20min to make the mixture even. The reaction solution was placed in an oil bath at 100°C for 2 hours and then stopped heating; after the precipitate was cooled to room temperature, the obtained precipitate was centrifuged and washed 3 times with deionized water, dried at 50°C for 2 hours to obtain sea urchin-shaped iron oxyhydroxide α-FeOOH.
[0034] The obtained sea urchin-shaped iron oxyhydroxide α-FeOOH was heated from room temperature to 350°C in a muffle furnace at a heating rate of 5°C / min and calcined for 3 hours to obtain sea urchin-shaped α-FeOOH 2 o 3 ;
[0035] Mix the obtained sea urchin-shaped iron oxyhydroxide α-FeOOH with ferrous salt (ferrous sulfate heptahydrate) at a molar ratio of 2:1, and react at...
Embodiment 2
[0037] Example 2: Preparation of three-dimensional ordered arrays of iron oxyhydroxide, ferric oxide, and ferric oxide
[0038] Weigh 30g FeSO 4 ·7H 2 Dissolve O in 100mL of deionized water, stir to dissolve completely, then add 10mL of ethylene glycol to it, and continue to stir for 20min to make the mixture even. The reaction solution was placed in an oil bath at 100°C for 2 hours and then stopped heating. After the precipitate was cooled to room temperature, the obtained precipitate was centrifuged and washed 3 times with deionized water, and dried at 50°C for 2 hours to obtain a three-dimensional ordered array of iron oxyhydroxide α-FeOOH .
[0039] The obtained iron oxyhydroxide α-FeOOH three-dimensional ordered array was heated from room temperature to 350°C in a muffle furnace at a heating rate of 5°C / min and calcined for 3h to obtain α-Fe 2 o 3 three-dimensional ordered array;
[0040] Mix the obtained iron oxyhydroxide α-FeOOH three-dimensional ordered array with...
Embodiment 3
[0042] Embodiment 3: Preparation of rod-shaped iron oxyhydroxide, ferric oxide, ferric oxide
[0043] Weigh 20g FeSO 4 ·7H 2 Dissolve O in 100mL deionized water, stir to dissolve completely, then add 10mL ethylene glycol to it, and continue stirring for 20min to make the mixture even. The reaction solution was placed in an oil bath at 120°C for 2 hours, and then the heating was stopped; after the precipitate was cooled to room temperature, the obtained precipitate was centrifuged and washed 3 times with deionized water, and dried at 50°C for 2 hours to obtain rod-shaped iron oxyhydroxide α-FeOOH.
[0044] The obtained rod-shaped iron oxyhydroxide α-FeOOH was heated from room temperature to 300°C in a muffle furnace at a heating rate of 5°C / min and calcined for 3 hours to obtain rod-shaped α-Fe 2 o 3 ;
[0045] Mix the obtained rod-shaped iron oxyhydroxide α-FeOOH with ferrous salt (ferrous sulfate heptahydrate) at a molar ratio of 2:1, and react at 100°C for 1 h at a pH va...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com