Application of mon-carbonyl curcumin compound-based visual PH fluorescent probe
A single carbonyl turmeric and fluorescent probe technology, applied in the field of detection, can solve problems such as mechanical wear, and achieve the effects of good selectivity, little fluorescence background interference, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
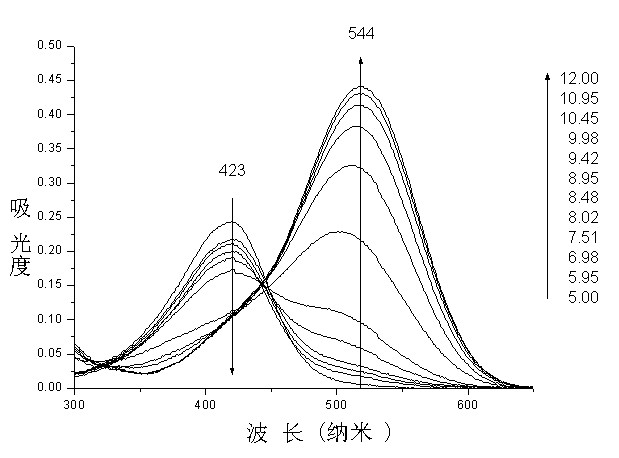
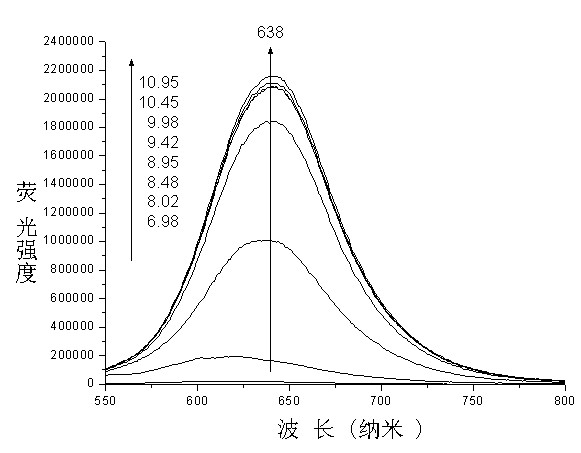
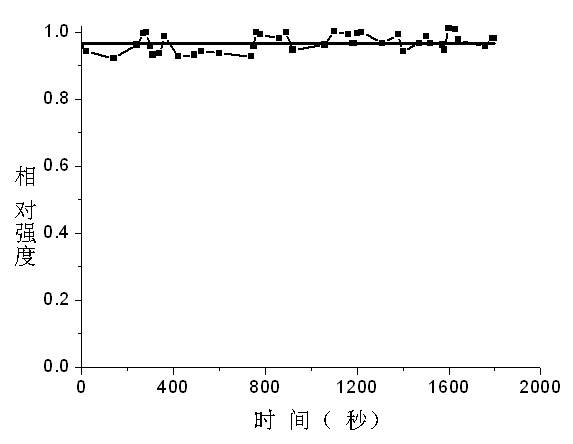
Image
Examples
Embodiment 1
[0028] Put 0.01 mol of 4-hydroxy-3-methoxy-benzaldehyde and 0.005 mol of acetone in a round bottom flask, add 20 mL of saturated HCl in glacial acetic acid solution, and stir at room temperature (25-30 ℃) for 30 min Leave it for 2 days. After the reaction was complete, 20 mL of water was added to the reaction flask, and the precipitate was filtered to obtain a crude product, which was recrystallized with ethanol and dried in vacuo to obtain an orange-yellow powder. 1,5-Bis(4-hydroxy-3-methoxy)-1,4-pentadien-3-one Yield 98%. mp 99–100 °C. 1 H NMR (DMSO-d6, 300 MHz) δ (ppm): 9.60 (brs, 2H, –OH), 7.63 (d, J = 15.9 Hz, 2H, –CH=C–), 7.35 (s, 2H, ArH ), 7.18 (d, J = 8.1 Hz, 2H, ArH), 7.12 (d, J = 15.9 Hz, 2H, –C=CH–), 6.81 (d, J = 8.1 Hz, 2H, ArH), 3.84 ( s, 6H, –OCH 3 ). ESI–MS (m / z): 325[M-1] - . Anal. Calc. for C 19 h 18 o 5 : C 69.93, H 5.56. Found: C 69.79, H 5.67.
Embodiment 2
[0030] Put 0.01 mol of 4-hydroxy-3-methoxy-benzaldehyde and 0.005 mol of cyclopentanone in a round-bottomed flask, add 20 mL of saturated HCl in glacial acetic acid, and stir at room temperature (25-30 °C) for 30 Leave it for 2 days after min. After the reaction was complete, 20 mL of water was added to the reaction flask, and the precipitate was filtered to obtain a crude product, which was recrystallized with ethanol. 2,5-Bis(4-hydroxy-3-methoxybenzylidene)cyclopentanone Yield 94%. mp 212–214 °C. 1H NMR (DMSO-d6, 300 MHz) δ (ppm): 9.64 (brs, 2H, –OH), 7.34 (s, 2H, –CH=), 7.23 (s, 2H, aroma), 7.15 (d, J = 8.1 Hz, 2H, ArH), 6.87 (d, J = 8.1 Hz, 2H, ArH), 3.83 (s, 6H, –OCH 3 ), 3.06 (s, 4H, –H 2 C–CH 2 –). ESI–MS (m / z): 351[M-1] - . Anal. Calc. for C 21 h 2 0O 5 : C 71.58, H 5.72. Found: C 71.50, H 5.88.
Embodiment 3
[0032] Put 0.01 mol of 4-hydroxy-3-methoxy-benzaldehyde and 0.005 mol of cyclopentanone in a round-bottomed flask, add 20 mL of saturated HCl in glacial acetic acid, and stir at room temperature (25-30 °C) for 30 Leave it for 2 days after min. After the reaction was complete, 20 mL of water was added to the reaction flask, and the precipitate was filtered to obtain a crude product, which was recrystallized with ethanol. Yield 98%. mp 178–179 °C. 1 H NMR (DMSO-d6, 300 MHz) δ (ppm): 9.48 (brs, 2H, –OH), 7.53 (s, 2H, –CH=), 7.08 (s, 2H, ArH), 7.01 (d, J = 8.1 Hz, 2H, ArH), 6.82 (d, J = 8.1 Hz, 2H, ArH), 3.79 (s, 6H, –OCH 3 ), 2.87 (t, J = 6.7 Hz, 4H, –H 2 C–C–CH 2 –), 1.71 (q, J = 6.7 Hz, 2H, –C–CH 2 –C–). ESI–MS (m / z): 365[M-1] - . Anal. Calc. for C 22 h 22 o 5 : C72.12, H 6.05. Found: C 72.03.48, H 6.02.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com