Method for capturing sulfur dioxide by employing imidazolyl ionic liquid
A sulfur dioxide, ionic liquid technology, applied in separation methods, chemical instruments and methods, dispersed particle separation, etc., can solve the problems of by-products, absorbent not easy to regenerate, small absorption capacity, poor cycle performance, etc., to avoid hydrogen bond networks. The effect of forming, improving absorption capacity and reducing viscosity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] In a 5ml glass container with an inner diameter of 1cm, add the ionic liquid tetradecyl trihexylphosphorus triazole ([P 66614 ][Tetz]) 1.2g (0.02mol), then slowly introduce sulfur dioxide gas, the flow rate is 60ml / min, the pressure is 0.1MPa, the absorption temperature is controlled at 20°C, and the absorption time is controlled at 0.5 hours. The weighing shows that the sulfur dioxide in the ionic liquid The absorption capacity is 3.72 mol / mol ionic liquid.
Embodiment 2-7
[0016] Similar to Example 1, the sulfur dioxide gas pressure was controlled to 0.1 MPa, the absorption temperature was 20°C, and the type of ionic liquid was changed. The results of sulfur dioxide absorption are shown in the following table (Table 1):
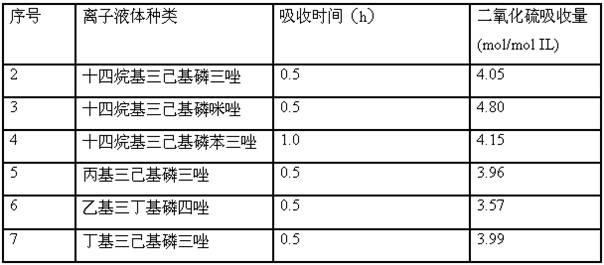
[0017] Table 1 Effects of different ionic liquids on SO2 capture
[0018]
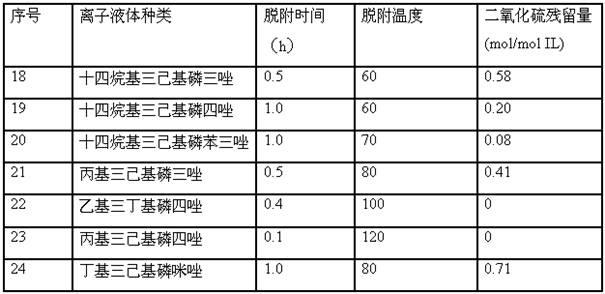
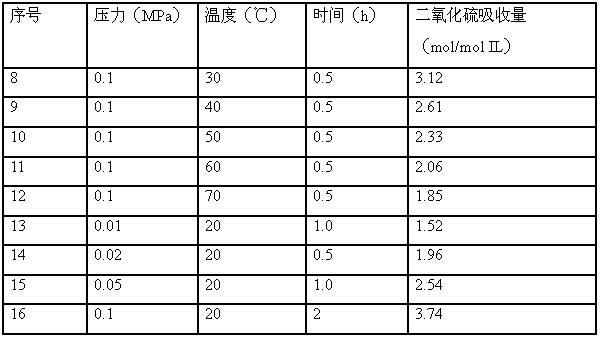
Embodiment 8-16
[0020] Similar to Example 1, tetradecyltrihexylphosphorus tetrazole was used as absorbent to absorb sulfur dioxide gas, and conditions such as absorption temperature, gas pressure, and absorption time were changed. The absorption results are shown in the following table (Table 2):
[0021] Table 2 Effect of different absorption conditions on SO2 absorption
[0022]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com