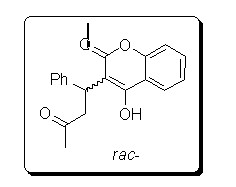
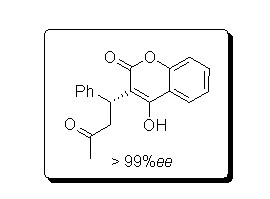
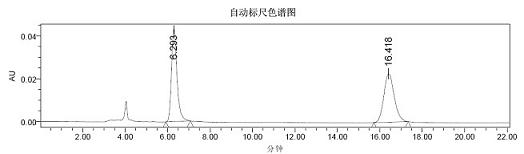
Preparation method of chiral warfarin and chiral warfarin derivatives
A derivative and chiral technology, which is applied in the field of preparation of coumarin anticoagulants—chiral warfarin and its derivatives, can solve the problem of weakening effect, increasing coagulation effect, failing to obtain good experimental results, etc. problem, to achieve the effect of low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1) Preparation of intermediate product substituted benzylidene acetone. Reaction formula:
[0044]
[0045] Steps:
[0046] A 50ml single neck round bottom flask was charged with benzaldehyde (0.02mol) and acetone (0.56mmol). Then add 0.5ml of 10% NaOH aqueous solution, then add 2ml of H 2 O. Stir at room temperature for 3h. After the reaction, the pH was adjusted with dilute HCl to make the solution neutral. Separation and purification (vacuum distillation) to obtain a viscous oily liquid, after cooling to obtain a pale yellow solid-benzylidene acetone (150 ~ 160 ℃ / 3333Pa (7mmHg)).
[0047] 2) Preparation of chiral warfarin. Reaction formula:
[0048]
[0049] Steps:
[0050] 10ml reaction test tube, add 4-hydroxycoumarin (0.1m mol), benzylidene acetone (0.12m mol), chiral primary amine catalyst (5m mol%), lithium perchlorate (5mmol%), Acetic acid (1mmol, 10eq), and finally add 2ml of 1,4-dioxane. Stir at room temperature for 24 hours, and quench by adding water after th...
Embodiment 2
[0061] 1) Preparation of intermediate product 4-methylbenzylideneacetone, reaction formula:
[0062]
[0063] Steps:
[0064] A 50ml single neck round bottom flask was charged with 4-methylbenzaldehyde (0.02mol) and acetone (0.56mmol). Then add 0.5ml of 10% NaOH aqueous solution, then add 2ml of H 2 O. Stir at room temperature for 3h. After the reaction, the pH was adjusted with dilute HCl to make the solution neutral. Separation and purification (vacuum distillation) to obtain a viscous oily liquid, after cooling to obtain a pale yellow solid-benzylidene acetone (150-160°C / 3333Pa(7mmHg)).
[0065] 2) Preparation of chiral warfarin derivatives, reaction formula:
[0066]
[0067] Steps:
[0068] 10ml reaction test tube, add 4-hydroxycoumarin (0.1621g, 0.1mmol, 1eq), intermediate product 4-methylbenzylideneacetone (0.220g, 0.12mmol, 1.2eq), chiral primary amine catalyst in one go (5mmol%), lithium perchlorate (5mmol%), acetic acid (1mmol, 10eq), and finally add 2ml of 1,4-dioxane. ...
Embodiment 3
[0070] 1) Preparation of the intermediate product 2-methoxybenzylideneacetone. Reaction formula:
[0071]
[0072] Steps:
[0073] A 50ml single neck round bottom flask was charged with 2-methoxybenzaldehyde (0.02mol) and acetone (0.56mmol). Then add 0.5ml of 10% NaOH aqueous solution, then add 2ml of H 2 O. Stir at room temperature for 3h. After the reaction, the pH was adjusted with dilute HCl to make the solution neutral. Separate and purify the fractions (distilled under reduced pressure) to obtain a viscous oily liquid. After cooling, obtain a light yellow solid-benzylidene acetone (150-160℃ / 3333Pa(7mmHg))
[0074] 2) Preparation of chiral warfarin derivatives, reaction formula:
[0075]
[0076] (3);
[0077] Steps:
[0078] 10ml reaction test tube, add 4-hydroxycoumarin (0.1mmol), intermediate product 2-methoxybenzylideneacetone (0.12mmol), chiral primary amine catalyst (5mmol%), lithium perchlorate ( 5mmol%), acetic acid (1mmol, 10eq), and finally add 2ml of 1,4-dioxane. S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com