Refining method of rebamipide
A technology of rebamipide and its refining method, which is applied in the field of preparation of high-purity rebamipide, can solve problems such as difficulty in obtaining high-purity rebamipide and residual impurities, and achieve beautiful color, simple operation and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
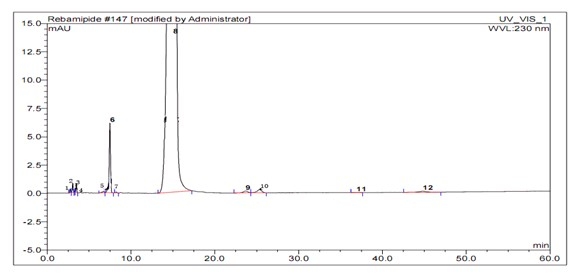
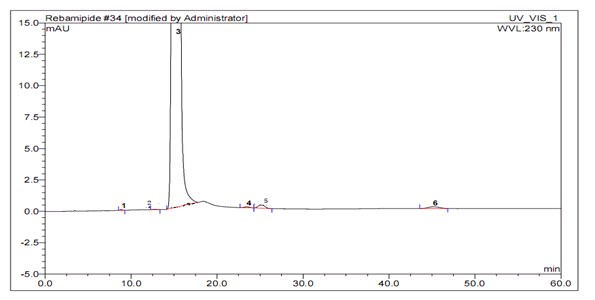
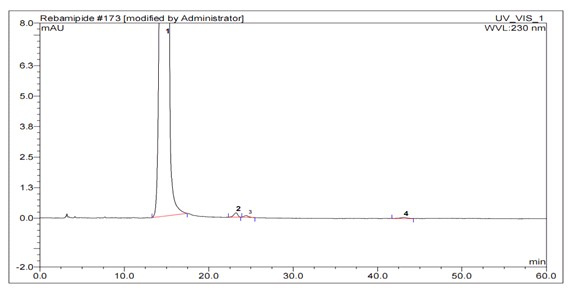
Image
Examples
Embodiment 1
[0033] 1) Preparation of crude rebamipide potassium salt
[0034] Put 370ml of methanol, 37g (0.10mol) of crude rebamipide, and 8.4g of potassium hydroxide into a 1000ml four-necked bottle in sequence, stir, and raise the temperature to 60~65°C to dissolve the solid. Add 2g of activated carbon, decolorize at this temperature for 1 hour, filter, and cool the filtrate to 0°C to crystallize. Filter, and dry the filter cake under reduced pressure at 60° C. for 8 hours to obtain 32 g of crude rebamipide potassium salt;
[0035] 2) Refined rebamipide potassium salt
[0036] Put 450ml of isopropanol and 32g of crude rebamipide potassium salt into a 500ml four-neck bottle, and heat up to 65°C to dissolve the solid. Add 2 g of activated carbon, reflux for decolorization for 1 hour, filter, and cool the filtrate to 0°C for crystallization. Filter, and dry the filter cake under reduced pressure at 60°C to obtain 29 g of refined rebamipide potassium salt;
[0037] 3) High-purity rebam...
Embodiment 2
[0040] 1) Preparation of crude product of rebamipide sodium salt
[0041] Put 550ml of methanol, 37g (0.10mol) of crude rebamipide, and 4.5g (0.143mol) of sodium hydroxide into a 1000ml four-necked bottle in sequence, stir, and raise the temperature to 75-80°C to dissolve the solid. Add 2g of activated carbon, decolorize at this temperature for 1 hour, filter, and cool the filtrate to 0°C to crystallize. Filter, and dry the filter cake under reduced pressure at 60°C to obtain 30 g of crude rebamipide sodium salt;
[0042] 2) Refined rebamipide sodium salt
[0043] Put 240ml of isopropanol, 120ml of ethyl acetate, and 30g of crude rebamipide sodium salt into a 500ml four-necked bottle, and raise the temperature to 75~80°C to dissolve the solid. Add 2 g of activated carbon, reflux for decolorization for 1 hour, filter, and cool the filtrate to 0°C for crystallization. Filter and dry the filter cake under reduced pressure at 60°C to obtain 27g of refined rebamipide sodium salt...
Embodiment 3
[0064] 1) Preparation of the crude product of rebamipide potassium salt
[0065] Put 300ml of isopropanol, 37g (0.10mol) of crude rebamipide, 13g of potassium carbonate and 60ml of water into a 1000ml four-necked bottle in turn, stir, and raise the temperature to 75~80°C to dissolve the solid. Add 2 g of activated carbon, decolorize at this temperature for 1 hour, filter, and cool the filtrate to 0 °C to crystallize 8. Filter, and dry the filter cake under reduced pressure at 60° C. to obtain 28 g of crude rebamipide potassium salt;
[0066] 2) Refined rebamipide potassium salt
[0067] Put 350ml of isopropanol and 28g of crude rebamipide potassium salt into a 500ml four-neck bottle, and raise the temperature to 75~80°C to dissolve the solid. Add 2 g of activated carbon for decolorization for 1 hour, filter, cool the filtrate to 0°C, crystallize, filter, and dry the filter cake under reduced pressure at 60°C to obtain 26 g of refined rebamipide potassium salt;
[0068] 3) H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com