Target protein for Alzheimer's disease and coding gene and application thereof
An Alzheimer's disease, protein technology, applied in the field of genetic engineering, can solve problems such as unclearness and Aβ accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: The Drosophila dZIP1 gene is responsible for Zn uptake in brain and body tissues
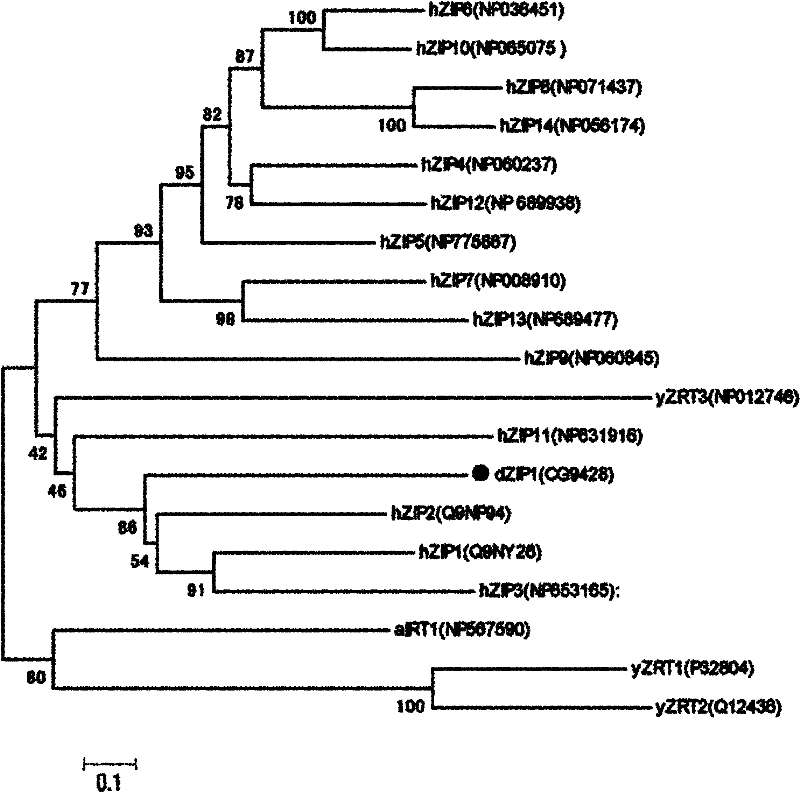
[0057] 1. Sequence analysis and comparison of human hZip1 ortholog gene dZip1 in Drosophila
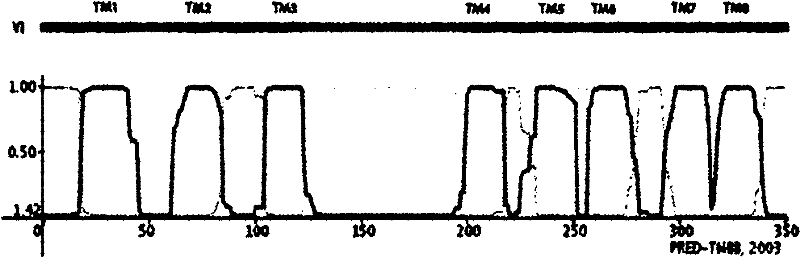
[0058] Using NCBI Blast software, Blast the amino acid sequence of the human Zip family gene hZip1 in the GenBank flybase database. As a result, the amino acid sequence of Drosophila melanogaster (Drosophila melanogaster) dZip1 (CG9428-PA) has the highest amino acid sequence identity with hZip1 (sequence identity = 29 %, E value = 2e-15). dZip1 is located on chromosome 2 of the Drosophila genome, and the dZip1 ORF region has 1135bp (including a 76bp Intron between 1018-1094bp), encoding 352aa, and the predicted molecular weight is 37836Da. Using TMHMM software to predict the transmembrane region of the dZip1 protein structure, it is shown that dZip1 has 8 typical TM domains of the Zip family, and contains a variable region between TM3 and TM4, which is located in the cytoplasm ( fig...
Embodiment 2
[0077] Example 2: Specific regulation of the expression level of dZip1 in the Drosophila nervous system can significantly affect the deposition of Aβ amyloid in the brain
[0078] Thioflavin-S (Thioflavin S, TS) staining can specifically identify Aβ fibers, which is used in this experiment to detect the deposition of Aβ42 fibers in the Drosophila brain. The specific operation is as follows. The Drosophila brain was first fixed in 4% paraformaldehyde and 2% Triton-X100 solution for 2 hours, and then transferred to 0.25% TS solution containing 50% ethanol overnight. Then wash with 50% ethanol once for 10 minutes, and wash with PBS three times for 10 minutes each time. After that, focusclear (Pacgen Biopharmaceuticals Co.) was added dropwise, and the coverslip was covered. The slices were observed with a Zeiss LSM 510 confocal laser microscope, and data analysis was performed with LSM 510 analysis software. TS-positive deposits in mushroom body regions of the Drosophila brain w...
Embodiment 3
[0079] Example 3: Changes in Aβ amyloid deposition in Drosophila brain are closely related to Zn balance changes caused by regulation of dZip1 expression level
[0080] 1. Real-time detection of dZip1 specific overexpression in the Drosophila nervous system and Zn accumulation in the brain caused by RNAi by Zinquin staining
[0081] Since Zinquin staining can specifically label Zn ions, this experiment uses this technique to detect the effect of different expression levels of dZip1 on Zn uptake in Drosophila brain in real time. The specific operation is as follows: Drosophila after eclosion for 10 days was transferred to the culture medium containing 4mM ZnCl 2 Drosophila brains were dissected at 40h and 72h respectively, and incubated in 25μM zinquin (Sigma) solution at 37°C for 30 minutes. Then, wash with PBS solution 3 times, 5 minutes each time. The treated Drosophila brain was observed and photographed under a fluorescent microscope (Nikon, Diaphot 300). the result sho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com