Application of Honokiol to preparing drugs for preventing or treating intracranial space occupying lesion and intracranial tissues and organs inflammation
A kind of technology with honokiol and uses, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment one honokiol injection
[0040] Prepared a kind of intravenous injection preparation of honokiol, formula is: honokiol 0.2%, Cremophor EL (polyoxyethylene castor oil) 1.5%, ethanol 1.5%, vitamin C 0.2%, EDTA disodium salt 0.03% , the water balance.
[0041] Preparation method: Accurately weigh the drug, add Cremophor EL and ethanol, stir until the drug is completely dissolved, add the aqueous solution of vitamin C and EDTA disodium salt, stir evenly, add water for injection to the specified amount, stir evenly until clear, and dilute hydrochloric acid or Adjust the pH to neutral with dilute sodium hydroxide. Filtration, potting, sterilization.
Embodiment 2
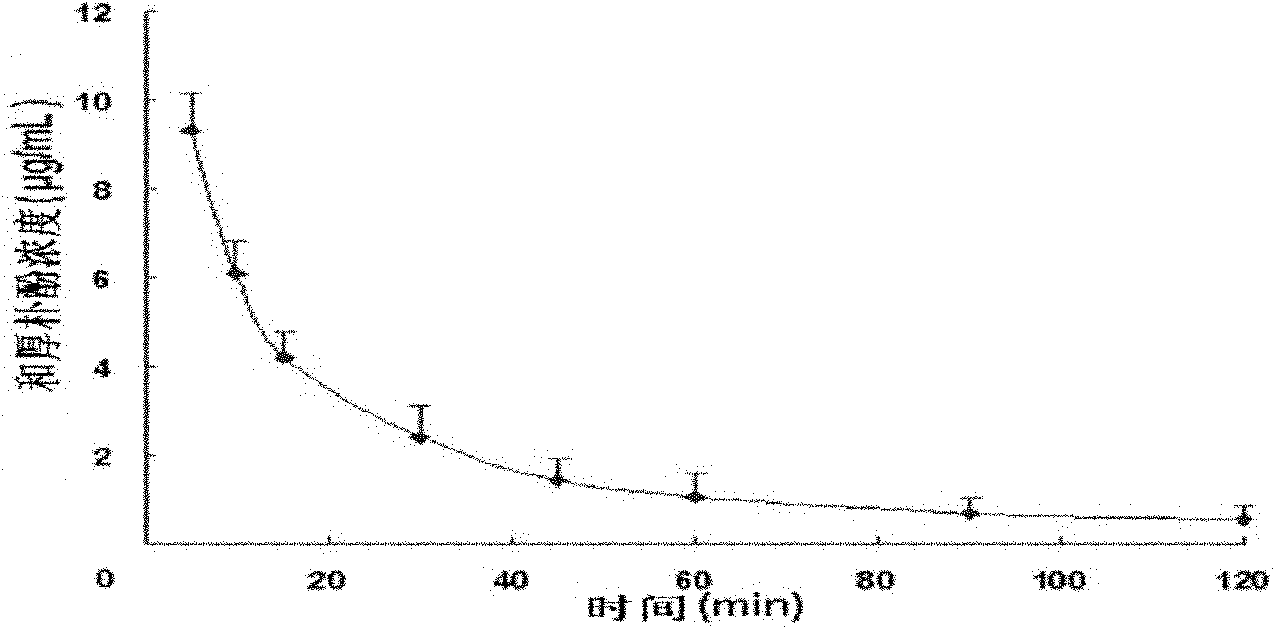
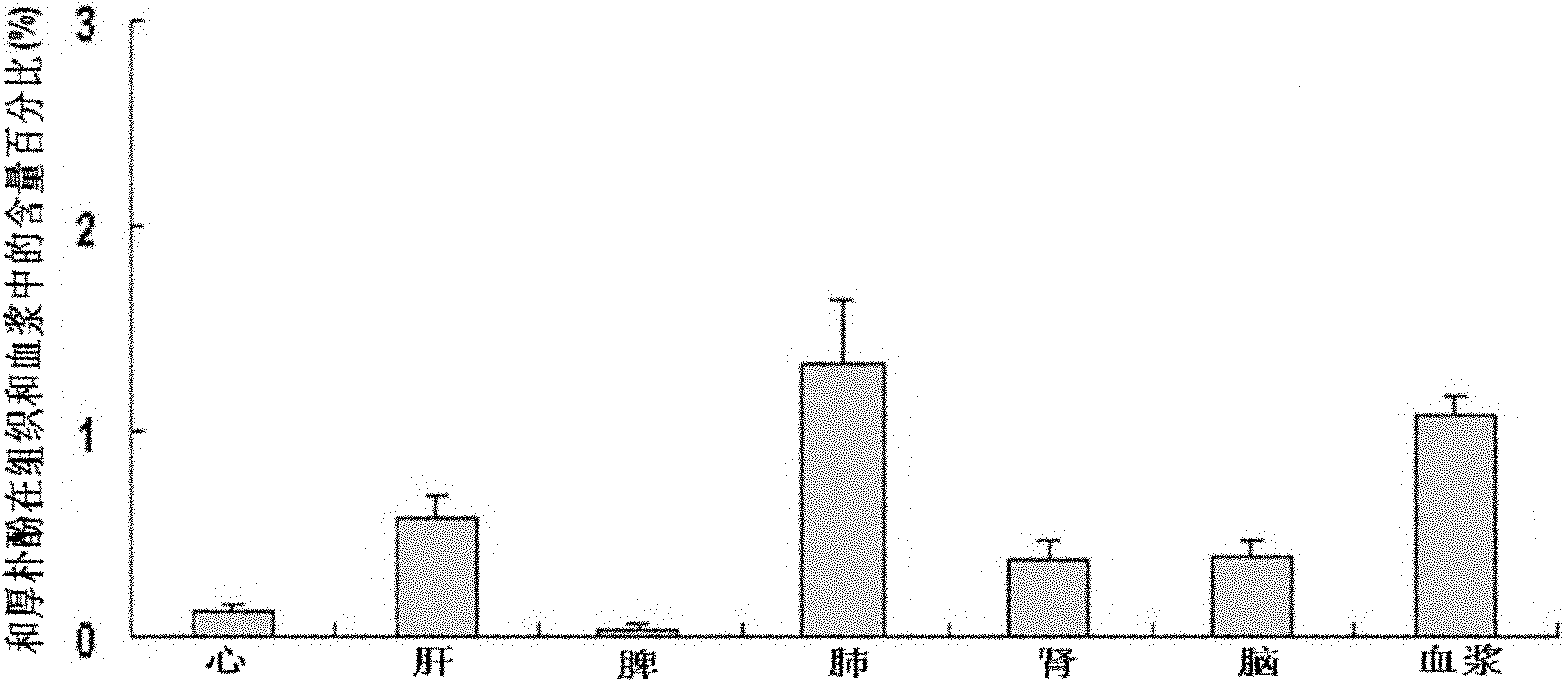
[0042] Example 2 Honokiol Injection Penetrates Blood Brain Barrier and Blood Cerebrospinal Fluid Barrier Test after Intravenous Administration
[0043] Laboratory method: 30 male SD rats with a body weight of 220-260g (from the West China Animal Room of Sichuan University), 6 for the determination of drug concentration-time curves in plasma, and another 24 for 5 minutes, 10 Minutes, 60 minutes and 120 minutes four time points (6 rats for each time point) drug concentration test in the brain and cerebrospinal fluid. After 6 rats were anesthetized, they were fixed in a supine position, and the right common carotid artery was intubated; the tail vein was given honokiol 20 mg / kg body weight, and the injection was prepared according to the magnolol injection in Example 1. Blood was collected at 0 minutes, 5 minutes, 10 minutes, 15 minutes, 30 minutes, 45 minutes, 60 minutes, 90 minutes and 120 minutes, and centrifuged at low temperature to obtain plasma. HPLC analysis drug concent...
Embodiment 3
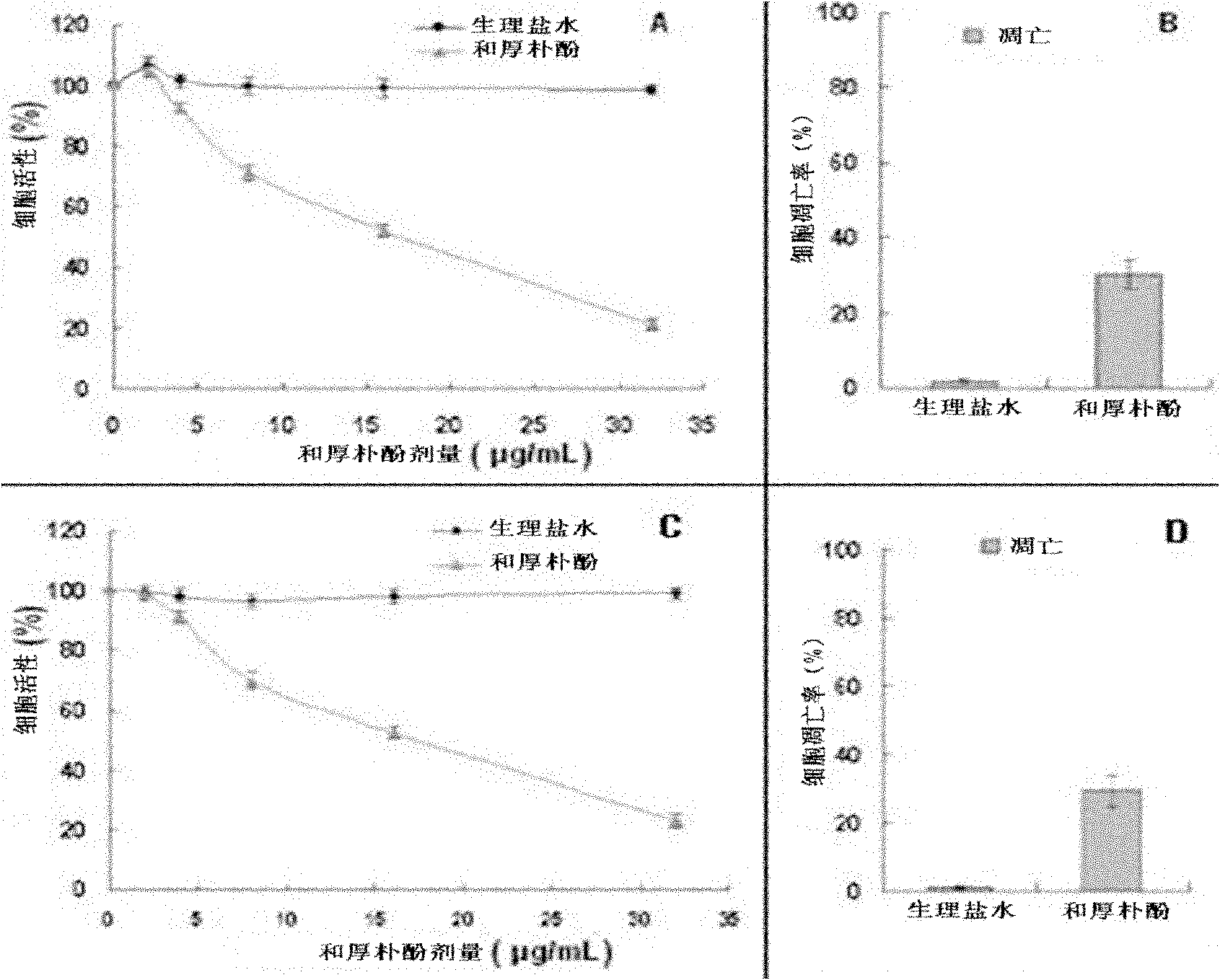
[0053] Example 3 In vitro proliferation test of honokiol inhibiting mouse 9L gliosarcoma cells and human U251 glioma cells
[0054] Laboratory method: Mouse 9L gliosarcoma cells and human U251 glioma cells were cultured in 96-well plates, and the plating density was 5×10 4 After the cells adhered to the wall, honokiol of different concentrations (0-32 μg / mL) was added, and the fresh DMEM medium was used as a blank. After incubation for 24 hours, the absorbance value was measured at 570 nm by the MTT method, and the IC was calculated. 50 . see results image 3 (A, C), the IC of honokiol on mouse 9L gliosarcoma cells 50 was 15.61μg / mL, while IC for human U251 glioma cells 50 It was 16.38 μg / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com