Idiotoxin solvent and preparation method thereof
A technology of allergens and solvents, which is applied in the direction of allergen antigen components, inorganic non-effective components, polymer compound non-effective components, etc., can solve the problems of loss, poor protein stability, and low effective active protein concentration, and achieve improved Effectiveness, improved stability, and improved clarity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1: Allergen Vehicle
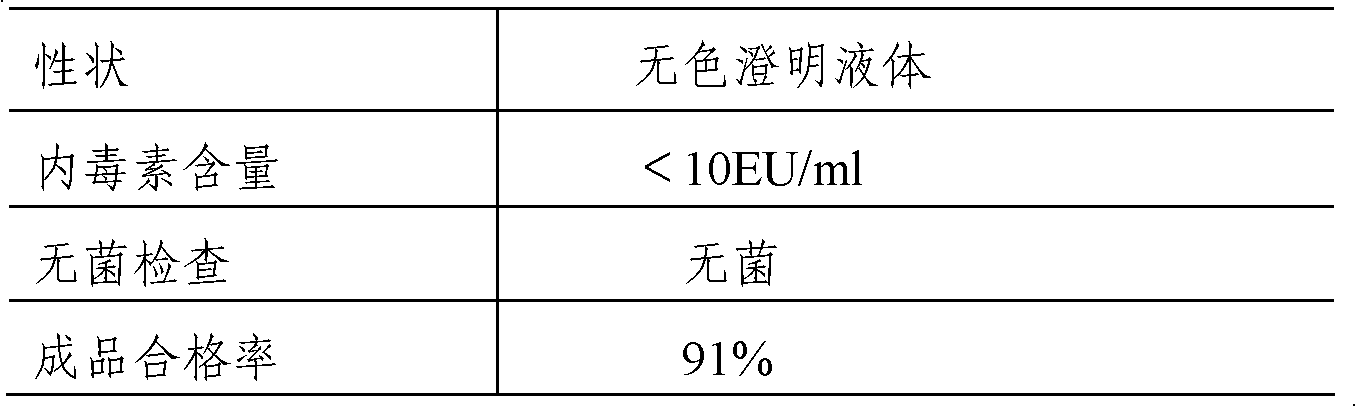
[0047] 1. Composition: 50mM disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution, 0.5g / 100ml sodium chloride, 0.4g / 100ml phenol, 0.03g / 100ml human serum albumin, pH8.0, the specification of the injection is 1.8ml / bottle.
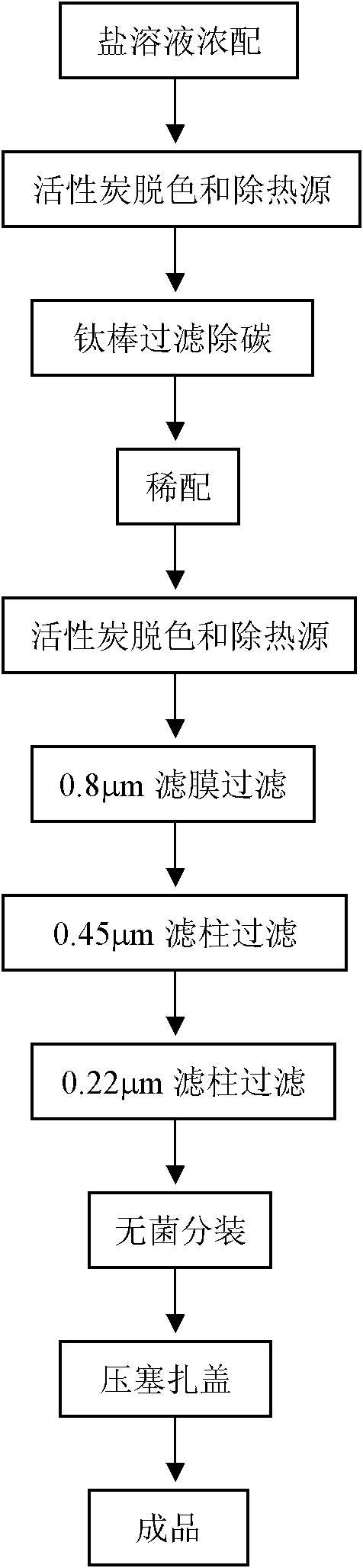
[0048] 2. Preparation method (such as figure 1 shown in the flowchart)
[0049] 1) Weigh 99.23g disodium hydrogen phosphate (0.699mol), 6.12g sodium dihydrogen phosphate (0.051mol), 75g sodium chloride and 60g phenol, dissolve in 2L 60°C water for injection, and dilute to 60°C water for injection 8L, spare;
[0050] 2) When the temperature drops to 30°C, add 0.2% activated carbon for needles, and stir at 30°C for 30 minutes;
[0051] 3) Filter the solution using a 1 μm titanium rod to remove carbon particles. The decarburized salt solution is adjusted to 14.5 L with 60°C injection water, and 0.05% activated carbon for needles is added, stirred and adsorbed at 60°C for 30 minutes, and filtered with a...
Embodiment 2
[0062] Example 2: Allergen Vehicle
[0063] 1. Composition: 50mM disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution, 0.5g / 100ml sodium chloride, 0.4g / 100ml phenol, 0.03g / 100ml human albumin, pH7.0, the specification of the injection is 1.8 ml / bottle.
[0064] 2. Preparation method:
[0065] 1) Weigh 72.85g disodium hydrogen phosphate (0.512mol), 28.44g sodium dihydrogen phosphate (0.237mol), 75g sodium chloride, 60g phenol, dissolve in 2L 60°C water for injection, and dilute to 60°C water for injection 8L;
[0066] 2) When the temperature drops to 30°C, add 0.2% activated carbon for needles, and stir at 30°C for 30 minutes;
[0067] 3) Filter the solution with a 1 μm titanium rod to remove carbon particles, and dilute the decarbonized salt solution to 14.5 L with 60°C water for injection; add 0.05% activated carbon for needles, stir and adsorb at 60°C for 30 minutes, and filter with a 0.8 μm membrane , to remove activated carbon;
[0068] 4) Add 22.5m...
Embodiment 3
[0072] Example 3: Allergen Vehicle
[0073] 1. Composition: 50mM disodium hydrogen phosphate-sodium dihydrogen phosphate buffer solution, 0.5% sodium chloride, 0.4% phenol, 0.03% human serum albumin, pH6.0, the specification is 1.8ml / bottle.
[0074] 2. Preparation method:
[0075] 1) Weigh 18.96g disodium hydrogen phosphate (0.134mol), 73.98g sodium dihydrogen phosphate (0.616mol), 75g sodium chloride, and 60g phenol, dissolve them in 2L 60°C water for injection, and make up to volume with 60°C water for injection up to 8L;
[0076] 2) When the temperature drops to 30°C, add 0.2% activated carbon for needles, and stir at 30°C for 30 minutes;
[0077] 3) Then use a 1 μm titanium rod to filter the solution to remove carbon particles, and the decarburized salt solution is adjusted to 14.5 L with 60°C injection water; add 0.05% activated carbon for needles, stir and adsorb at 60°C for 30 minutes, and filter with a 0.8 μm membrane , to remove activated carbon;
[0078] 4) Add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com