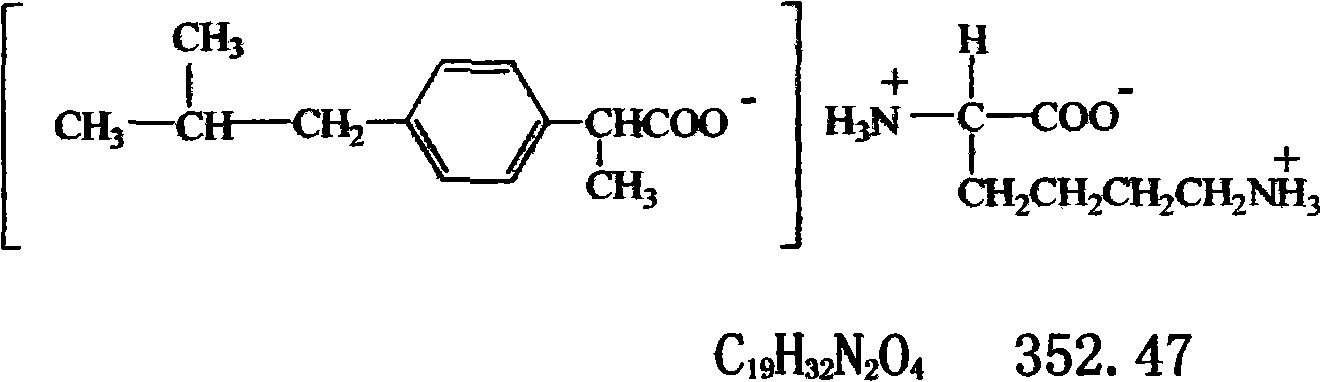Synthesis and application of ibuprofen lysine
A technology of lysine and synthesis process, which is applied in the field of lysinoprofen synthesis and its application, can solve the problems of poor water solubility of ibuprofen, limited preparation types and clinical applications, etc., and achieves strong tolerance and expanded preparation types. and clinical application, the effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: Under normal temperature and normal pressure, carry out the synthetic technique of first decolorization rear reaction:
[0019] Dissolve 16.4kg (0.1kmol) of L-type and DL-lysine monohydrate (generally, it should reach more than 98% according to the dry product) in 16.4kg, 49.2kg, 82kg, 114.8kg, and 164kg of water respectively to obtain five groups Solution A1, A2, A3, A4, A5; 20.6kg (0.1kmol) ibuprofen (generally should reach more than 98% by dry product) is dissolved in 20.6kg, 61.8kg, 103kg, 144.2kg, 206kg ethanol respectively (95% industrial ethanol is enough), get five groups of solutions B1, B2, B3, B4, B5. The molar feeding ratios are respectively 1.0:1; 1.1:1; 1.2:1; 1.3:1; 1.5:1. Add 1kg (according to the total weight of the solution to calculate the amount of activated carbon added) activated carbon to decolorize the above-mentioned five groups of solutions A and five groups of solutions B respectively, and filter. Then mix the two corresponding...
Embodiment 2
[0020] Embodiment 2: Under normal temperature and pressure, carry out the synthetic technique of decolorization after first reaction:
[0021] Dissolve 16.4kg (0.1kmol) DL-lysine monohydrate in 16.4kg, 49.2kg, 82kg, 114.8kg, 164kg water to obtain solutions A1, A2, A3, A4, A5; Ibuprofen was dissolved in 20.6kg, 61.8kg, 103kg, 144.2kg, and 206kg of ethanol to obtain solutions B1, B2, B3, B4, and B5. Mix solution A and solution B, react at 25°C for 2 hours, add 2kg of activated carbon for decolorization, filter, cool, add 75kg of ethanol, cool to -10°C for 10 hours to crystallize. Centrifuge, wash with ethanol, spin dry, and dry below 50°C to obtain 23.9kg of lysinoprofen, which is a white crystalline powder.
[0022] It should be noted:
[0023] 1. In the above two embodiments, not only DL-lysine monohydrate, but also D-lysine, L-lysine and their monohydrates can be used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com