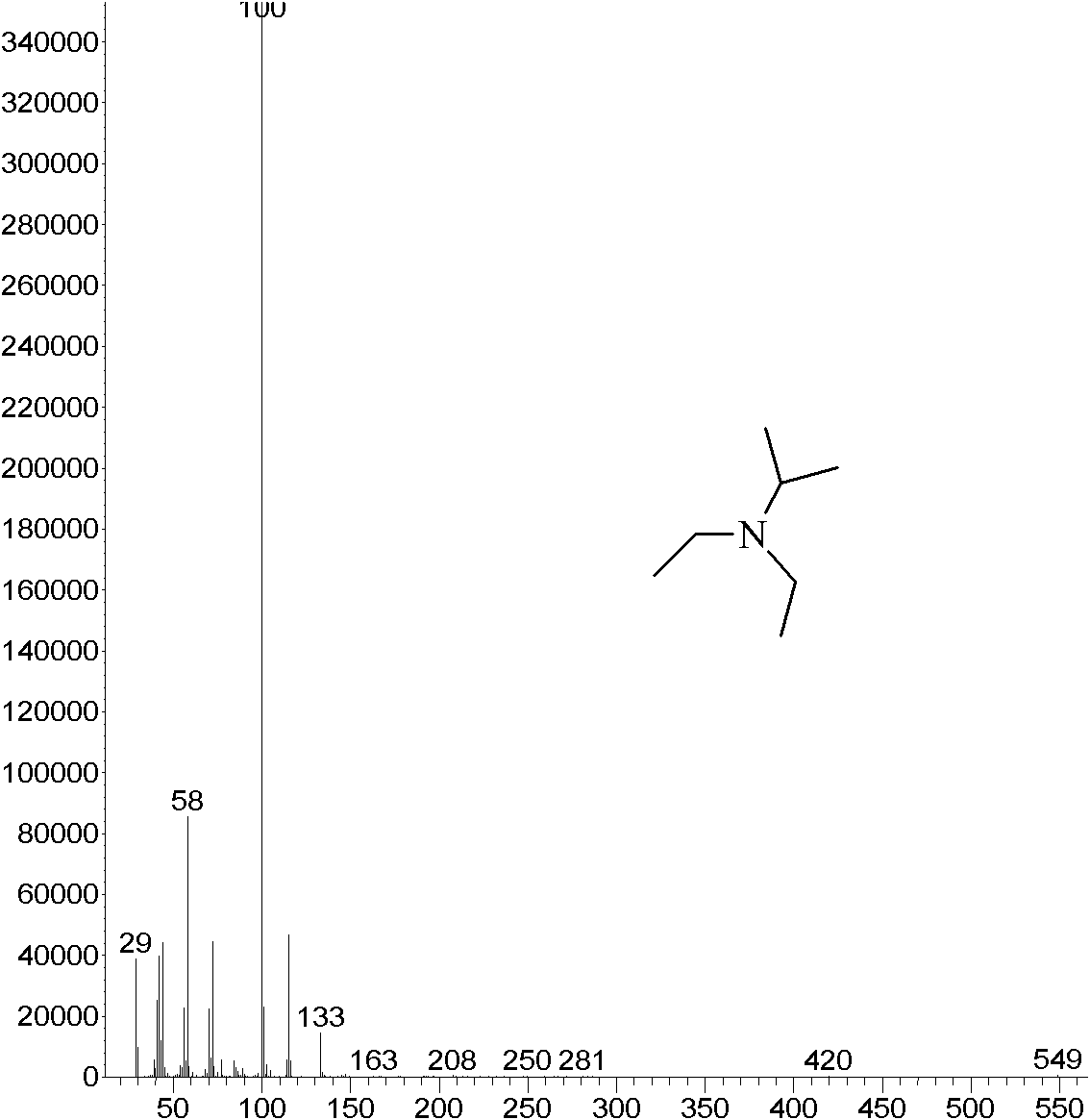
Synthesis method of N,N-diethyl isopropylamine
A technology of diethylisopropylamine and a synthetic method, which is applied in the preparation of amino compounds, chemical instruments and methods, and the preparation of organic compounds, can solve the problems that solvents increase the difficulty of product separation, it is difficult to realize industrial production, and the price of raw materials is expensive. Achieve the effect of low price, high utilization rate of raw materials, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1, a kind of N, N, the synthetic method of-diethylisopropylamine:
[0028] Add 58.6g (0.8mol) of diethylamine and 31.4g (0.4mol) of isopropyl chloride into the autoclave with a stirring temperature measuring device, and close the lid of the kettle. use N 2 After checking for leaks and replacing the air several times, the temperature was raised to 100°C and the pressure was 0.2MPa. The reaction was terminated after maintaining the temperature for 4 h.
[0029] Add NaOH aqueous solution (mass concentration: 10%) to the obtained reaction product until PH = 13.5, separate the upper organic phase for rectification, collect 107.5~108.0°C fractions, and obtain the product N,N,-diethylisopropylamine 36.4g, the yield is 79.2%, the purity is 99%, and the obtained product is characterized by the correct structure.
Embodiment 2
[0030] Embodiment 2, a kind of N, N, the synthetic method of-diethylisopropylamine:
[0031] Add 58.6g (0.8mol) of diethylamine and 31.4g (0.4mol) of isopropyl chloride into the autoclave with a stirring temperature measuring device, and close the lid of the kettle. use N 2 After checking for leaks and replacing the air several times, the temperature was raised to 150°C and the pressure was 0.6MPa. The reaction was terminated after maintaining the temperature for 6 h.
[0032] Add KOH aqueous solution (mass concentration: 20%) to the obtained reaction product until PH = 13.5, separate the organic phase located in the upper layer for rectification, collect fractions at 107.5~108.0°C, and obtain the product N,N,-diethyliso Propylamine was 37.4g, the yield was 81.2%, and the purity was 99%. The resulting product was characterized and had a correct structure.
Embodiment 3
[0033] Embodiment 3, a kind of N, N, the synthetic method of-diethylisopropylamine:
[0034] Add 58.6g (0.8mol) of diethylamine and 31.4g (0.4mol) of isopropyl chloride into the autoclave with a stirring temperature measuring device, and close the lid of the kettle. use N 2 After checking for leaks and replacing the air several times, the temperature was raised to 140°C and the pressure was 0.5MPa. After maintaining the temperature for 8 hours, the reaction was terminated.
[0035] Add KOH aqueous solution (mass concentration: 30%) to the obtained reaction product until PH = 11.5, separate the organic phase located in the upper layer for rectification, collect fractions at 107.5~108.0°C, and obtain the product N,N,-diethyliso Propylamine was 39.3g, the yield was 85.4%, and the purity was 99%. The resulting product was characterized to have a correct structure.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com