Three-dimensional micro-nano material composed of nano CoFe2O4 and preparation method thereof
A micro-nano material, three-dimensional technology, applied in the direction of nanostructure manufacturing, nanotechnology, nanotechnology, etc., to achieve the effect of simple and easy process, easy to master, and good reaction reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] 1. Preparation of precursors
[0036] Add ferric salt and divalent cobalt salt with a total concentration of 25-35mM and a molar ratio of 2:1 to ethylene glycol, stir it electromagnetically to become a transparent liquid, heat the oil bath to 200-230°C, and react for 50-70 minutes , cooled to room temperature, and collected the precipitate. The precipitate is centrifuged and washed four times with ethanol, and dried at 60-80° C. for 6-8 hours to obtain a cobalt ferrite precursor.
[0037] 2. Target product
[0038] The precursor is heated from room temperature to 350°C for 2-3 hours in a tube furnace with an air flow rate of 50-60ml / min at a heating rate of 20-30°C / min to obtain the target product.
Embodiment 1
[0040] (1) Preparation of precursors
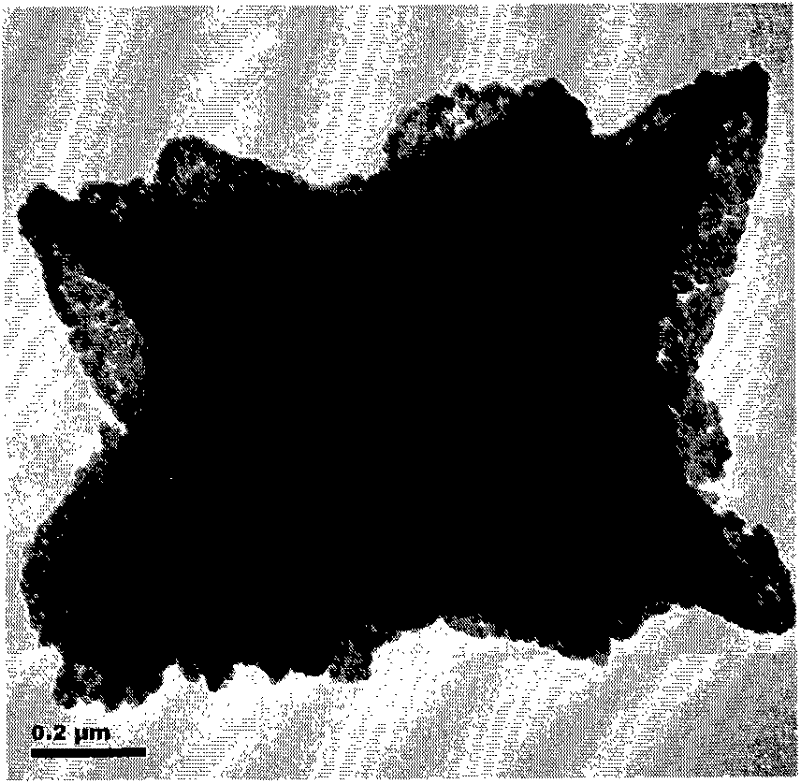
[0041] Add iron and cobalt nitrates with a total concentration of 30 mM into 180 mL of ethylene glycol at a molar ratio of 2:1, stir electromagnetically, heat the oil bath to 230° C., and stop the reaction after 60 minutes. Cool to room temperature, centrifuge and wash the precipitate 4 times with absolute ethanol, and dry it in a drying oven at 80°C for 6 hours to obtain the precursor, the CoFe 2 o 4 Large-scale SEM photographs of precursors such as figure 1 As shown, its high-resolution SEM photos are shown as figure 2 shown.
[0042] (2)CoFe 2 o 4 preparation of
[0043] The air in the tube furnace is 60ml / min, and at a heating rate of 30°C / min, the precursor is burned from room temperature to 350°C for 2 hours to obtain the monodisperse target product CoFe 2 o 4 , the CoFe 2 o 4 The side and front TEM photographs of image 3 and Figure 4 shown.
[0044] The CoFe prepared in this embodiment 2 o 4 The XRD pattern of F...
Embodiment 2
[0047] (1) Preparation of precursors
[0048] Add ferric trichloride and cobalt dichloride with a total concentration of 30 mM to 180 mL of ethylene glycol at a molar ratio of 2:1, stir electromagnetically, heat the oil bath to 230° C., and stop the reaction after 60 minutes. After cooling to room temperature, the precipitate was centrifuged and washed 4 times with absolute ethanol, and dried in a drying oven at 80° C. for 6 hours to obtain the precursor.
[0049] (2)CoFe 2 o 4 preparation of
[0050] The air in the tube furnace is 60ml / min, and at a heating rate of 30°C / min, the precursor is burned from room temperature to 350°C for 2 hours to obtain the monodisperse target product CoFe 2 o 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com