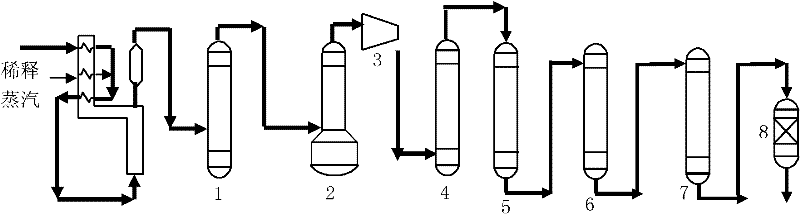
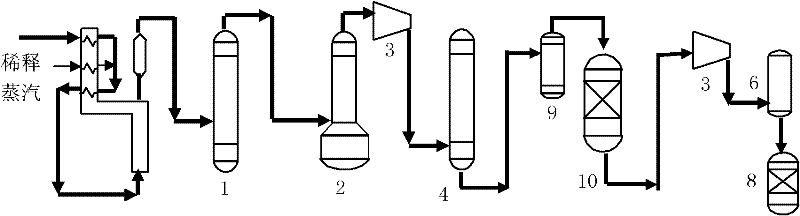
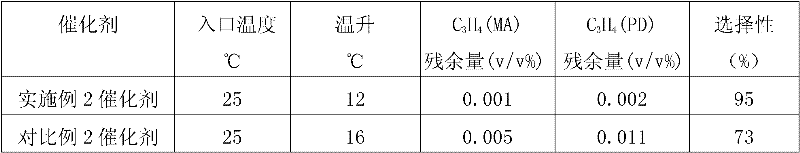
Selective hydrogenation method for C3 fraction
A technology for selective hydrogenation and fractionation. It is applied in the fields of hydrocarbons, chemical instruments and methods, and hydrocarbon purification/separation. It can solve problems such as increasing the difficulty of operation, improve safety, reduce the risk of overheating, and improve Effect of liquid phase volumetric space velocity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Catalyst preparation:
[0061] A. Functionalized polyvinyl chloride (PVC) / Al 2 o 3 preparation of
[0062] Weigh Φ4.5mm, length is 4.5mm, specific surface area is 100m 2 / g, columnar Al with a pore volume of 0.60ml / g 2 o 3 Carrier 250g.
[0063] Dissolve 10g of PVC completely in 400ml of tetrahydrofuran (THF), impregnate the above-mentioned carrier into the above-mentioned solution, stir and let stand to deposit PVC on Al 2 o 3 Surface, dry. Get 260gPVC / Al 2 o 3 product.
[0064] Add 80g of dicyandiamide to 500ml of distilled water, stir to dissolve completely, then add 260g of PVC / Al 2 o 3 Reflux for 120min, cool to room temperature, wash with deionized water until neutral, and dry to obtain functionalized PVC / Al 2 o 3 . In moles, the moles of complexing agent dicyandiamide / moles of reactive groups Cl in the macromolecule=59.5.
[0065] B. (Pd-Ag)-polymer / Al 2 o 3 Precursor preparation
[0066] Weigh 1.65gPd(NO 3 ) 2 , 0.8gAgNO 3 , measure 0.5ml o...
Embodiment 2
[0083] Catalyst preparation:
[0084] A. Functionalized polystyrene acrylonitrile (SAN) / Al 2 o 3 preparation of
[0085] Weigh Φ2.5mm, the specific surface area is 50m 2 / g, the pore volume is 0.38ml / g 3 spherical Al 2 o 3 Carrier 250g.
[0086] Weigh 1.1g of SAN resin, dissolve it in 300ml of dimethylformamide (DMF) solvent, stir at room temperature to completely dissolve the SAN resin, add the above-mentioned weighed carrier in this solution, let it stand for 60min after fully stirring, and separate the solvent After drying, SAN / Al 2 o 3 .
[0087] The above obtained SAN / Al 2 o 3 , added to 500ml of deionized water, 28.8g of ethylenediamine was added, refluxed for 300min, the product was taken out after cooling, washed until neutral, and dried to obtain functionalized SAN / Al 2 o 3 . In terms of moles, the number of moles of complexing agent ethylenediamine / the number of moles of reactive groups CN in the macromolecule=23.96.
Embodiment 3
[0106] Catalyst preparation:
[0107] A. Preparation of functionalized polyvinyl chloride (PVC)
[0108] Weigh 5g of PVC and completely dissolve it in 400ml of THF, add 40g of dicyandiamide, reflux for 120min, cool to room temperature, and obtain functionalized PVC for later use after washing. In terms of moles, the number of moles of complexing agent dicyandiamide / the number of moles of reactive Cl groups in the macromolecule=5.94.
[0109] B. (Pd-Ag)-polymer complex / Al 2 o 3 Precursor preparation
[0110] Weigh Φ4.5mm, length is 4.5mm, specific surface area is 15m 2 / g, the pore volume is 0.3ml / g 3 Columnar Al 2 o 3 Carrier 250g.
[0111] Weigh 1.75gPd(NO 3 ) 2 , 0.24gAgNO 3 , Measure 6ml of nitric acid, add to the above functionalized PVC solution, stir for 5min to obtain (Pd-Ag)-PVC.
[0112] 250g Al 2 o 3 The carrier was added into the mixed solution, and after being fully stirred and allowed to stand for 1 h, the above product was washed with deionized wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com