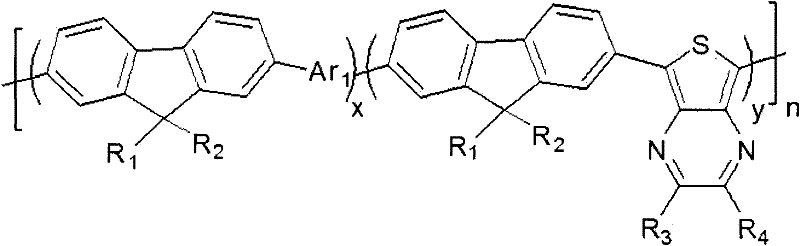
Thieno (3,4-b) pyrazine contained fluorene copolymer, preparation method and application thereof
A 4-b, copolymer technology, applied in the direction of electrical components, semiconductor/solid-state device manufacturing, electric solid-state devices, etc., can solve the problems of rare and limited material development, and achieve improved stability, wide spectral response range, and reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] A fluorene copolymer of the following molecular structural formula:
[0058]
[0059] The preparation steps of above-mentioned fluorene copolymer are as follows:
[0060] (1) Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dihexylfluorene:
[0061]
[0062] Add 13.00mL (2.00M) of n-butyllithium solution to a reaction flask containing 4.92g of 2,7-dibromo-9,9-dihexylfluorene and 100.00mL of tetrahydrofuran at -78°C under nitrogen. After stirring for 2.5 hours, slowly add 5.50 mL of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane dropwise, return to room temperature, and continue stirring for 26 hours . After the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, washed with petroleum ether, and precipitated with methanol to obtain white needle crystals. MALDI-TOF-MS (m / z): 586.5 (M + ).
[0063] (2) Preparation of 2,5-dibromothieno[3,2-b]t...
Embodiment 2
[0073] This embodiment discloses a fluorene copolymer with the following structure:
[0074]
[0075] The preparation steps of above-mentioned fluorene copolymer are as follows:
[0076] (1) Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dioctylfluorene:
[0077]
[0078] At -78°C under nitrogen, add 22.00mL (2.00M) of n-butyllithium solution to two ports containing 11.00g of 2,7-dibromo-9,9-dioctylfluorene and 80.00mL of tetrahydrofuran with a syringe In the flask, after stirring for 2 hours, slowly add 10.00mL 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane dropwise, return to room temperature, continue Stir for 29 hours. After the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, washed with petroleum ether, and precipitated with methanol to obtain white needle crystals. MALDI-TOF-MS (m / z): 642.6 (M + ).
[0079] (2) Preparation of 2,5-dibr...
Embodiment 3
[0089] This embodiment discloses a fluorene copolymer with the following structure:
[0090]
[0091] The preparation steps of above-mentioned fluorene copolymer are as follows:
[0092] (1) Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolyl)-9,9-dihexylfluorene:
[0093]
[0094] Add 13.00mL (2.00M) of n-butyllithium solution to a reaction flask containing 4.92g of 2,7-dibromo-9,9-dihexylfluorene and 100.00mL of tetrahydrofuran at -58°C under nitrogen. After stirring for 2.5 hours, slowly add 5.50 mL of 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane dropwise, return to room temperature, and continue stirring for 21.5 hours . After the reaction was completed, the reaction solution was poured into water, extracted with ether, dried over anhydrous magnesium sulfate, rotary evaporated, washed with petroleum ether, and precipitated with methanol to obtain white needle crystals. MALDI-TOF-MS (m / z): 586.5 (M + ).
[0095] (2) Preparation of 2,5-dibromothi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com