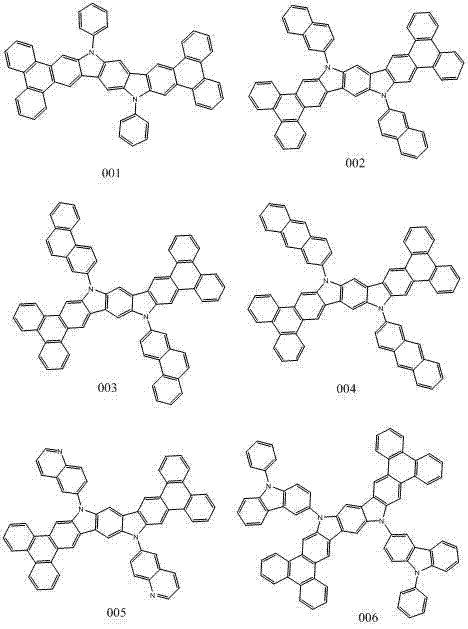
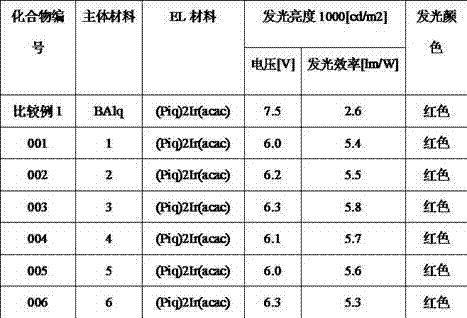
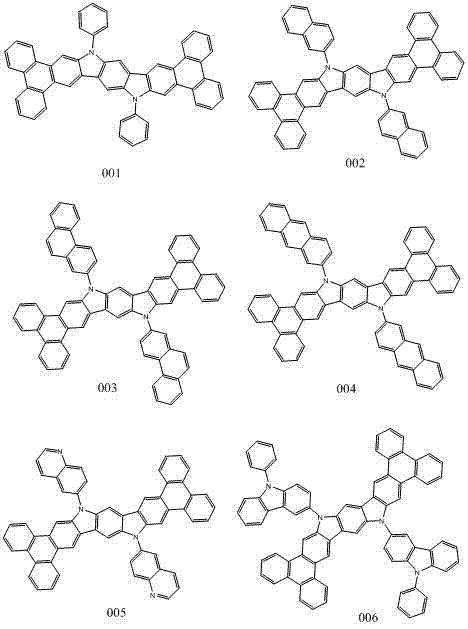
Novel organic electroluminescent compound and organic electroluminescent device comprising same
A technology of luminescent materials and derivatives, applied in organic chemistry, electrical solid devices, electrical components, etc., can solve the problems that cannot meet the requirements of OLED use, and meet the needs of industrial development, with simple synthesis and purification processes and low cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035]
[0036] 10,16-dihydroindolo[3,2-B]phenanthrene[9,10-H]carbazole 7.19mmol, bromobenzene 21.65mmol, sodium tert-butoxide 30.18mmol, tris(dibenzylideneacetone) 400 mg of dipalladium and 100 mg of tri-tert-butylphosphine were dissolved in 60 ml of toluene, under nitrogen protection, and stirred at 90° C. for 3 hours. After the reaction was completed, cool to room temperature, extract three times with dichloromethane and water, evaporate the organic phase to dryness with anhydrous magnesium sulfate, and obtain 3.56 g of indolo[2,3-H]carbazole derivatives by column chromatography. 70%. Mass Spectrum: Calculated 708.86; Found 708.82. Elemental analysis: Calculated value: C: 91.50; H: 4.55; N: 3.95; Tested value: C: 91.53; H: 4.56; N: 3.91. 1 H NMR(500MHz,Chloroform) δ9.86(s,1H), 9.58(s,1H), 9.06(s,1H), 7.72(d,J=3.5Hz,1H), 7.68 (s,1H), 7.65 (dd, J=7.3, 3.5Hz, 1H), 7.63–7.55(m, 4H), 7.49 (d, J=10.0Hz, 4H).
Embodiment 2
[0038]
[0039] 10,16-dihydroindolo[3,2-B]phenanthrene[9,10-H]carbazole 7.19mmol, 2-bromonaphthalene 21.65mmol, sodium tert-butoxide 30.18mmol, tri(dibenzylidene Dissolve 400 mg of acetone) dipalladium and 100 mg of tri-tert-butylphosphine in 60 ml of toluene, under nitrogen protection, and stir at 90° C. for 3 hours. After the reaction was completed, cool to room temperature, extract three times with dichloromethane and water, evaporate the organic phase to dryness with anhydrous magnesium sulfate, and obtain 3.56 g of indolo[2,3-H]carbazole derivatives by column chromatography. 70%. Mass Spectrum: Calcd. 808.98; Tested 808.96. Elemental analysis: Calcd: C: 92.05; H: 4.49; N: 3.46; Tested: C: 92.03; H: 4.48; N: 3.49.
Embodiment 3
[0041]
[0042]10,16-dihydroindolo[3,2-B]phenanthrene[9,10-H]carbazole 7.19mmol, 2-bromophenanthrene 21.65mmol, sodium tert-butoxide 30.18mmol, tri(dibenzylidene Dissolve 400 mg of acetone) dipalladium and 100 mg of tri-tert-butylphosphine in 60 ml of toluene, under nitrogen protection, and stir at 90° C. for 3 hours. After the reaction was completed, cool to room temperature, extract three times with dichloromethane and water, evaporate the organic phase to dryness with anhydrous magnesium sulfate, and obtain 3.56 g of indolo[2,3-H]carbazole derivatives by column chromatography. 70%. Mass Spectrum: Calculated 909.10; Found 909.13. Elemental analysis: Calcd: C: 92.48; H: 4.44; N: 3.08; Tested: C: 92.46; H: 4.45; N: 3.10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com