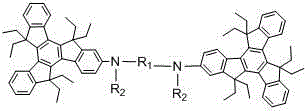
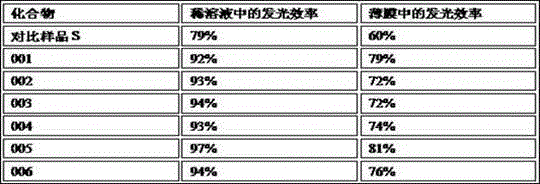
Novel electroluminescent material and applications thereof
A technology of electroluminescent materials and luminescent materials, applied in the direction of luminescent materials, condensation/addition reactions to prepare amino compounds, chemical instruments and methods, etc., can solve the problems that luminescent materials cannot meet the requirements of OLEDs, and achieve a good planar structure Effects of conjugated system, life improvement, and solubility improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
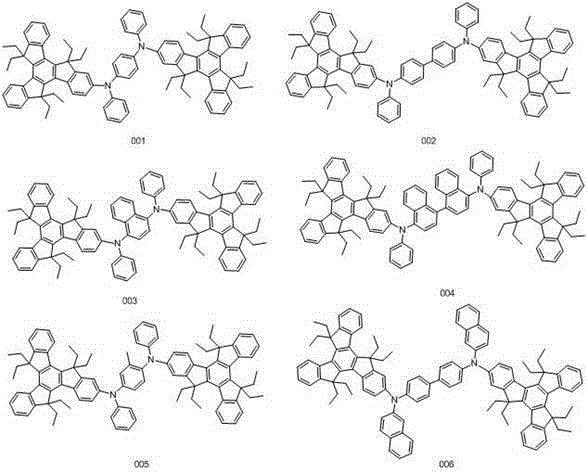
[0034] Embodiment 1: the synthesis of compound 001
[0035] The specific synthetic route is shown in the following formula:
[0036]
[0037] Under the condition of nitrogen protection, add 10.81g of phenylenediamine, 47.10g of bromobenzene, 0.75g of tris(dibenzylideneacetone)dipalladium, 2,2,-bis(diphenylphosphine)-1,1 into a 300ml single-necked bottle ,-binaphthyl (BINAP) 1.05g, potassium tert-butoxide 10.5g, dehydrated toluene 200ml, react at 85°C for 6 hours. After cooling, the reaction solution was filtered, and the obtained crude product was purified by silica gel chromatography, and dried under reduced pressure to obtain 24.73 g of a white solid intermediate.
[0038] 168.05g of 2-bromo-5,5,10,10,15,15-hexaethyl-10,15-dihydro-5H-indenofluorene, 24.73g of white solid intermediate, 21.32g of potassium tert-butoxide, 1.02 g of palladium (II) acetate, 0.97 g of tri-tert-butylphosphine, and 250 ml of dehydrated toluene were reacted at 85°C for 10 hours. The reaction so...
Embodiment 2
[0040] Embodiment 2: the synthesis of compound 002
[0041] The specific synthetic route is shown in the following formula:
[0042]
[0043] Under the condition of nitrogen protection, add 18.40g of phenylenediamine, 47.10g of bromobenzene, 1.50g of tris(dibenzylideneacetone)dipalladium, 2,2,-bis(diphenylphosphine)-1, 2.1g of 1,-binaphthyl (BINAP), 21.1g of potassium tert-butoxide, and 200ml of dehydrated toluene were reacted at 86°C for 7 hours. After cooling, the reaction solution was filtered, and the obtained crude product was purified by silica gel chromatography, and dried under reduced pressure to obtain 30.27 g of a white intermediate.
[0044] 173.65g of 2-bromo-5,5,10,10,15,15-hexaethyl-10,15-dihydro-5H-indenofluorene, 30.27g of white intermediate, 22.44g of potassium tert-butoxide, acetic acid 1.08 g of palladium (II), 1.01 g of tri-tert-butylphosphine, and 250 ml of dehydrated toluene were reacted at 86°C for 11 hours. The reaction solution was filtered, and ...
Embodiment 3
[0046] Embodiment 3: the synthesis of compound 003
[0047] The specific synthetic route is shown in the following formula:
[0048]
[0049] Under the condition of nitrogen protection, add 15.82g of naphthalene diamine, 47.10g of bromobenzene, 1.50g of tris(dibenzylideneacetone)dipalladium, 2,2,-bis(diphenylphosphine)-1,1 into a 300ml one-port bottle 2.1 g of -binaphthyl (BINAP), 21.1 g of potassium tert-butoxide, and 200 ml of dehydrated toluene were reacted at 86°C for 6 hours. After cooling, the reaction solution was filtered, and the obtained crude product was purified by silica gel chromatography, and dried under reduced pressure to obtain 28.56 g of a white intermediate.
[0050] 2-Bromo-5,5,10,10,15,15-hexaethyl-10,15-dihydro-5H-indenofluorene 179.25g, white intermediate 28.56g, potassium tert-butoxide 23.56g, acetic acid 1.14 g of palladium (II), 1.06 g of tri-tert-butylphosphine, and 250 ml of dehydrated toluene were reacted at 87°C for 12 hours. The reaction s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com