Bi-thiophene silole-containing conjugated polymer and preparation method and application thereof
A technology of conjugated polymer and thiophene thiorole, which is applied in the field of organic compound synthesis, can solve the problems of low collection efficiency of carrier electrodes, ineffective use of red light region, low carrier mobility, etc., and achieve excellent electrical conductivity. Effects of chemical reduction properties, enhanced reduction potential, improved transport properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
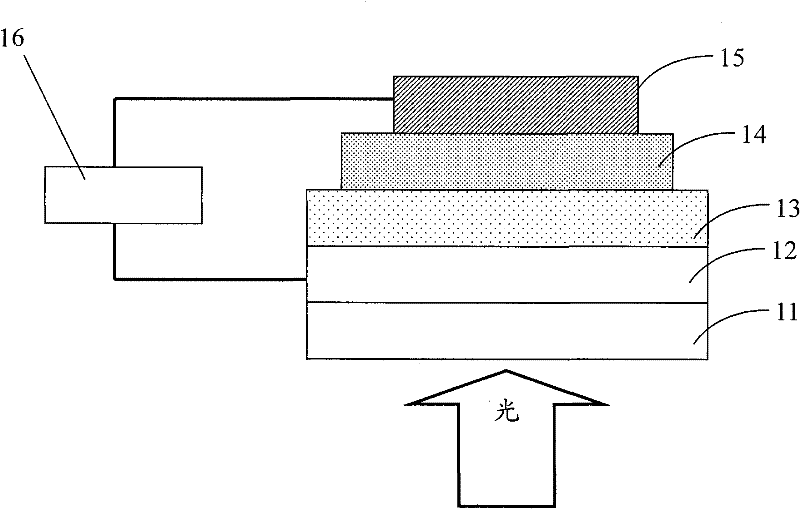
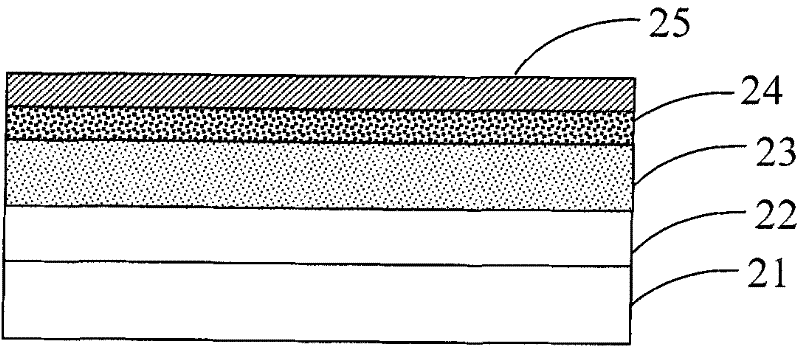
Image
Examples
preparation example Construction
[0039] And, the embodiment of the present invention also provides the preparation method of the conjugated polymer containing dithiophene thiazoles, including the following chemical reaction formula:
[0040]
[0041] That is, the specific process steps included are:
[0042] (1) Compounds A, B, C, diketones and 3,4-diaminothiophene hydrogen chloride represented by the following structural formula are provided respectively,
[0043]
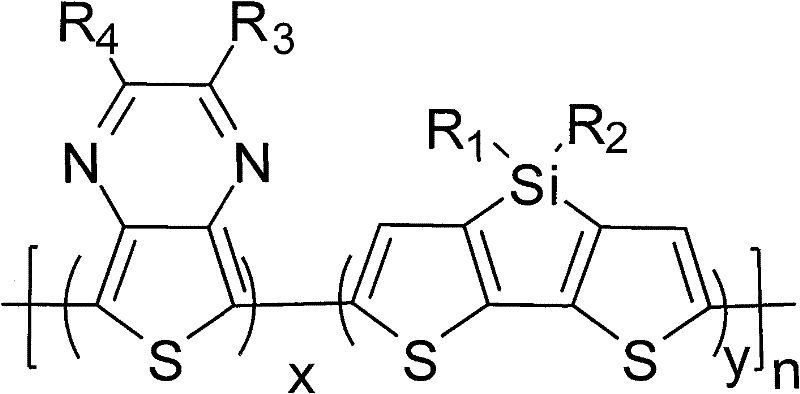
[0044] In the formula, any positive integer of x+y=1, 01 , R 2 from C 1 ~C 15 the alkyl group, R 3 , R 4 selected from -H, C 1 ~C 15 Alkyl, C 1 ~C 15 At least one of the alkoxy groups, alkyl or / and alkoxythiophene groups, alkyl fluorene groups, alkyl carbazole groups, and alkyl benzene ring groups;
[0045] (2) Under weak alkalinity and 0-78°C, the molar ratio of diketone compound and 3,4-diaminothiophene hydrogen chloride is 1:0.1-10 for dehydration reaction for 1-24h to obtain 2, 3-disubstituted-thieno[3,4-b]pyrazine;
[0046] ...
Embodiment 1
[0059] The following structural formula P 1 Polymer Synthesis:
[0060]
[0061] (1) 2, the preparation of 3-bisphenyl-thieno [3,4-b] pyrazine, its reaction is shown in the following formula:
[0062]
[0063] The specific process of preparation is: 3,4-diaminothiophene hydrochloride (1.0g, 5.34mmol) was added to the compound containing diphenyl ketone (1.12g, 5.34mmol) and triethylamine (10.68mmol) without To a solution of water and ethanol (100 mL), triethylamine (1.15 g, 11 mmol) was added and stirred at 78° C. for 1 hour under the condition of protecting from light. After the reaction, the solvent was removed by rotary evaporation of the reaction liquid, and a solid was obtained by column chromatography (dichloromethane as eluent). MS (EI) m / z: 288 (M + ).
[0064] (2) 5,7-dibromo 2, the preparation of 3-bisphenyl-thieno[3,4-b]pyrazine, its reaction is shown in the following formula:
[0065]
[0066] The specific process of preparation is: NBS (0.72g, 4mmol)...
Embodiment 2
[0071] The following structural formula P 2 Polymer Synthesis:
[0072]
[0073] (1) 2, the preparation of 3-bisphenyl-thieno [3,4-b] pyrazine, its reaction is shown in the following formula:
[0074]
[0075] The specific process of preparation is: 3,4-diaminothiophene hydrogen chloride (5.34mmol) is added to anhydrous ethyl acetate (100mL) solution of compound diphenylethanedione (0.534mmol) and sodium carbonate (26.7mmol) , then added triethylamine (1.15 g, 11 mmol) and stirred in an ice bath for 24 hours under light-shielding conditions, then the solvent was removed by rotary evaporation, and a solid was obtained by column chromatography (dichloromethane as eluent). MS (EI) m / z: 288 (M + ).
[0076] (2) 5,7-dibromo 2, the preparation of 3-bisphenyl-thieno[3,4-b]pyrazine, its reaction is shown in the following formula:
[0077]
[0078]The specific process of preparation is: NBS (0.72g, 4mmol) is added to tetrahydrofuran (THF) / N, N- Dimethylformamide (DMF) (v / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com