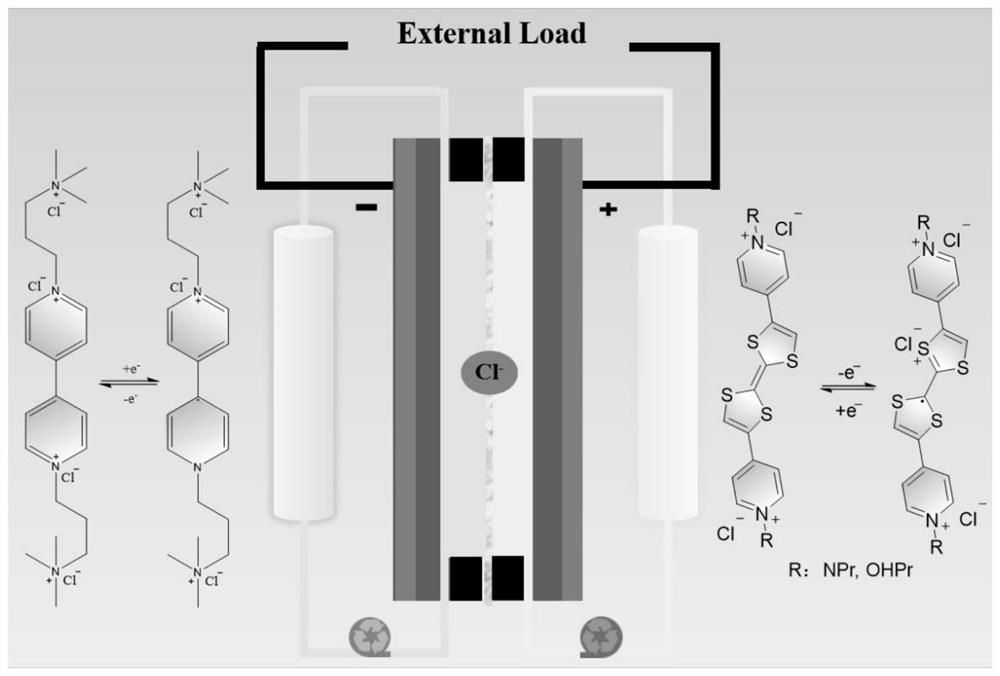
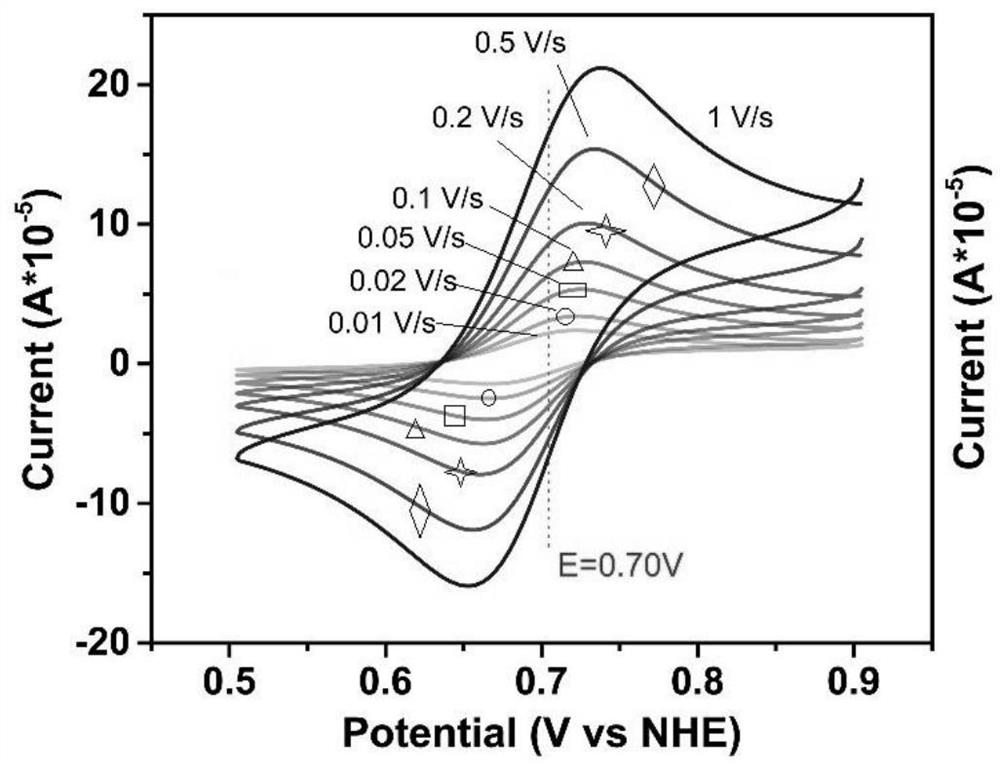
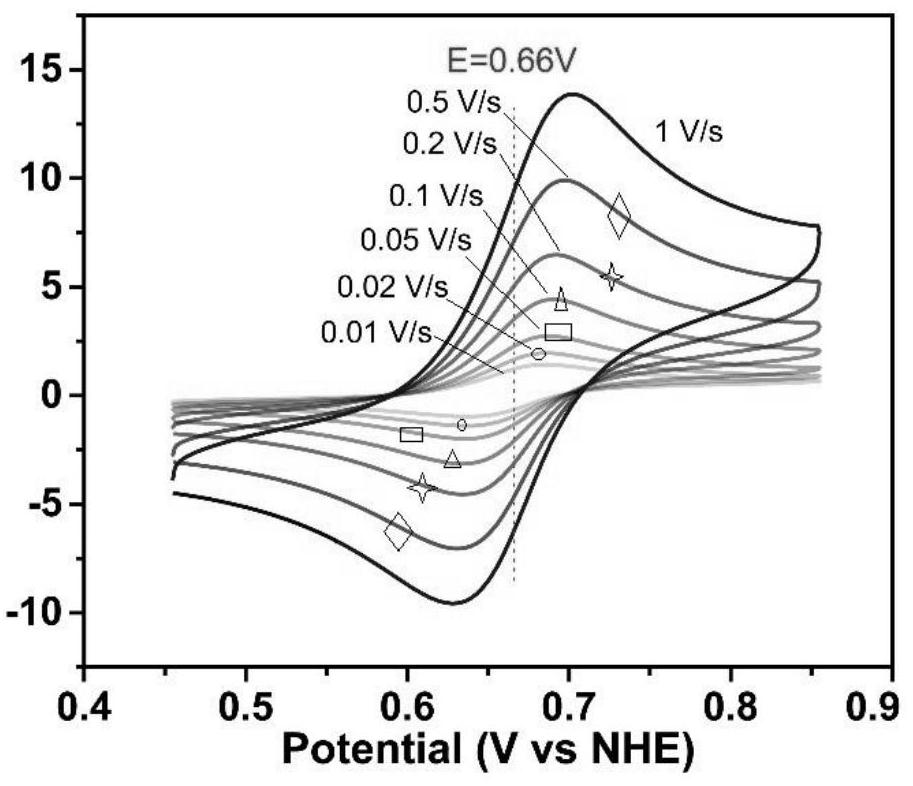
Tetrathiafulvalene-based viologen derivative as well as preparation method and application thereof in flow battery system
A technology of tetrathiafulvalene and derivatives, which is used in regenerative fuel cells, fuel cells, circuits, etc., to achieve the effects of improving molecular stability, broadening applications, and enriching species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The preparation method of a class of viologen derivatives based on tetrathiafulvalene of the present invention comprises the following steps:
[0057] Step 1: Bromide (C 7 h 6 Synthesis of BrNO HBr) (compound 1):
[0058] In the air, take 4-acetylpyridine (4-acetylpyridine 3mmol), HBr solution (containing HBr77mmol) with a mass fraction of 40%, and liquid bromine (32mmol) in a round-bottomed flask, and the molar ratio of the three is 1 : 25.7: 10.7, fully stirred, and fully reacted in an ice-water bath at 0-5°C for 1.5-2 hours.
[0059] Post-treatment process: quickly vacuum filter the reaction solution, wash with 2-5mL distilled water, the color of the product will gradually become lighter, transfer the obtained light yellow solid to a 100mL round-bottomed flask, and add a magnet to stir, 100mL Saturated sodium thiosulfate solution was transferred to a 250mL branch bottle, connected to a mechanical pump, a 100mL round bottom flask with product and a temperature cont...
Embodiment 1
[0081] 1, the preparation method of a class of viologen derivatives based on tetrathiafulvalene of the present invention comprises the following steps:
[0082] Step 1: Bromide (C 7 h 6 BrNO HBr) synthesis (compound 1);
[0083] Take a 50mL round bottom flask, wash and dry it for later use. Prepare plenty of ice cubes and 100 mL of saturated sodium thiosulfate solution in advance. Use a syringe to extract 3.63g (3mmol) 4-acetylpyridine (preserved at low temperature) into a round-bottomed flask, then place the round-bottomed flask in an ice-water bath, keep stirring, and then slowly add 10.90mL of HBr with a mass fraction of 40% The solution (containing HBr77mmol) forms a mixed solution. During this process, it should be added dropwise, the flow rate should be maintained at 1s / drop, and the stirring should be accelerated to prevent solidification. Use a long needle to draw 1.65mL of liquid bromine (32mmol) and quickly transfer it to a 10mL separatory funnel (remove the liqu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com