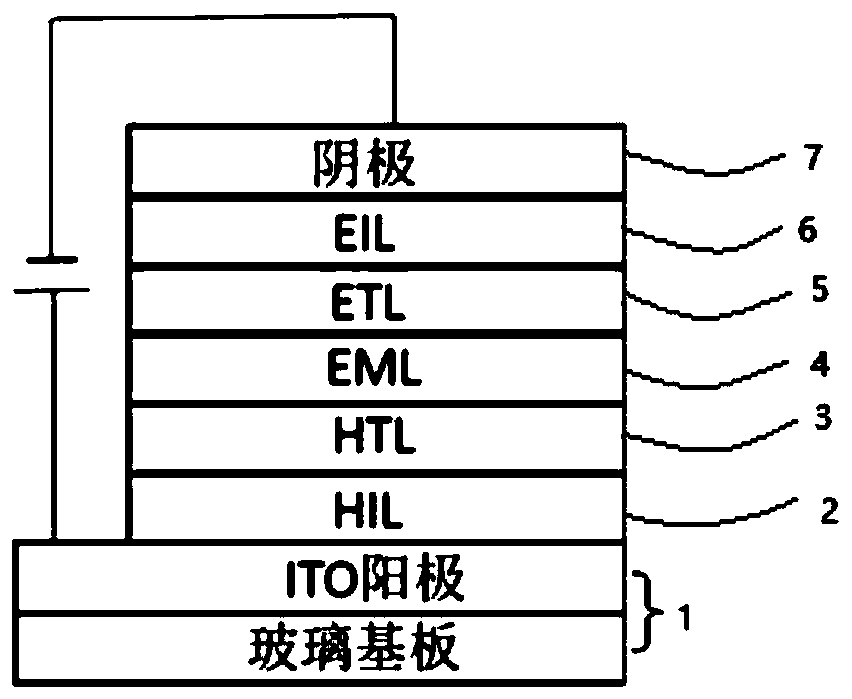
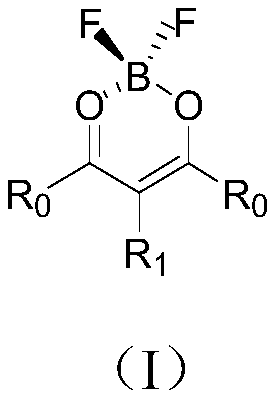
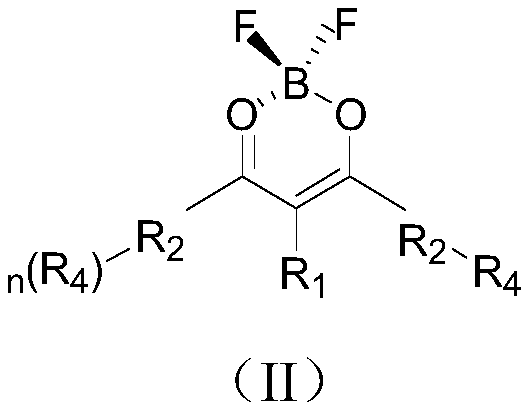
Organic compound and organic light emitting diode
An organic compound, light-emitting diode technology, applied in the field of organic functional materials, to achieve the effects of low efficiency, roll-off effect, and high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1: Preparation (I-1) of intermediate electron acceptor (A):
[0054]
[0055] In a nitrogen atmosphere, put 2g (5.25mmol) of 1,3-bis(4-bromophenyl)-3-hydroxyprop-2-en-1-one into a two-neck round bottom flask, add 100mL of dichloromethane to dissolve , Add 0.75g (5.25mmol) boron trifluoride ether with a syringe, heat to 60°C and reflux for 2h. Cool to room temperature, filter and spin dry. Recrystallized in acetone:n-hexane to obtain 1.76 g of solid, with a yield of 71%. 1H NMR 8.01 (d, 2H), 7.79 (d, 2H), 7.62 (s, 1H) 7.55 (d, 2H), 7.27 (d, 2H); MS (EA): m / e 427.9 (M+).
Embodiment 2
[0056] Embodiment 2: the preparation of organic compound 1
[0057]
[0058] Add 1.4 g of intermediate (I-1), 1.3 phenoxazine, 0.08 g of palladium acetate, 0.3 g of tri-tert-butylphosphine tetrafluoroborate, 0.7 g of sodium tert-butoxide and 30 mL of anhydrous toluene into a two-necked flask, After refluxing for 48h under the protection of nitrogen, it was cooled to room temperature, added dichloromethane to filter, and spin-dried. Pass through the column with petroleum ether:dichloromethane at a volume ratio of 1:2 to obtain 1.45 g of a brown-red product. Yield 71%. 1HNMR 7.64(d,2H),7.62(s,1H),7.13(d,2H),6.92-6.89(m,8H),6.82(d,2H),6.77(m,4H),6.59-6.58(m , 6H); MS (EA): m / e 664.4 (M+).
Embodiment 3
[0059] Embodiment 3: the preparation of organic compound 2
[0060]
[0061] Add the formula 1.3g intermediate (I-1), 1.3 phenothiazine, 0.08g palladium acetate, 0.3g tri-tert-butylphosphine tetrafluoroborate, 0.7g sodium tert-butoxide and 30mL anhydrous toluene into a two-necked flask , Refluxed for 48h under the protection of nitrogen, cooled to room temperature, added dichloromethane to filter, and spin-dried. Pass through the column with petroleum ether:dichloromethane at a volume ratio of 1:1 to obtain 1.21 g of a brown-red product. Yield 63%. 1 HNMR 7.64(d,2H),7.62(s,1H),7.21-7.16(m,12H),7.13(d,2H),6.97(m,4H),6.82(d,2H),6.58(d,2H ); MS (EA): m / e 696.2 (M+).
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com