Tetramine monomer and preparation method and application thereof
A monomer and polyimide technology, applied in the preparation of amino compounds, carbon-based compounds, chemical instruments and methods, etc., can solve the problem of poor thermal stability, film-forming thermal properties and mechanical properties of polyimide, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
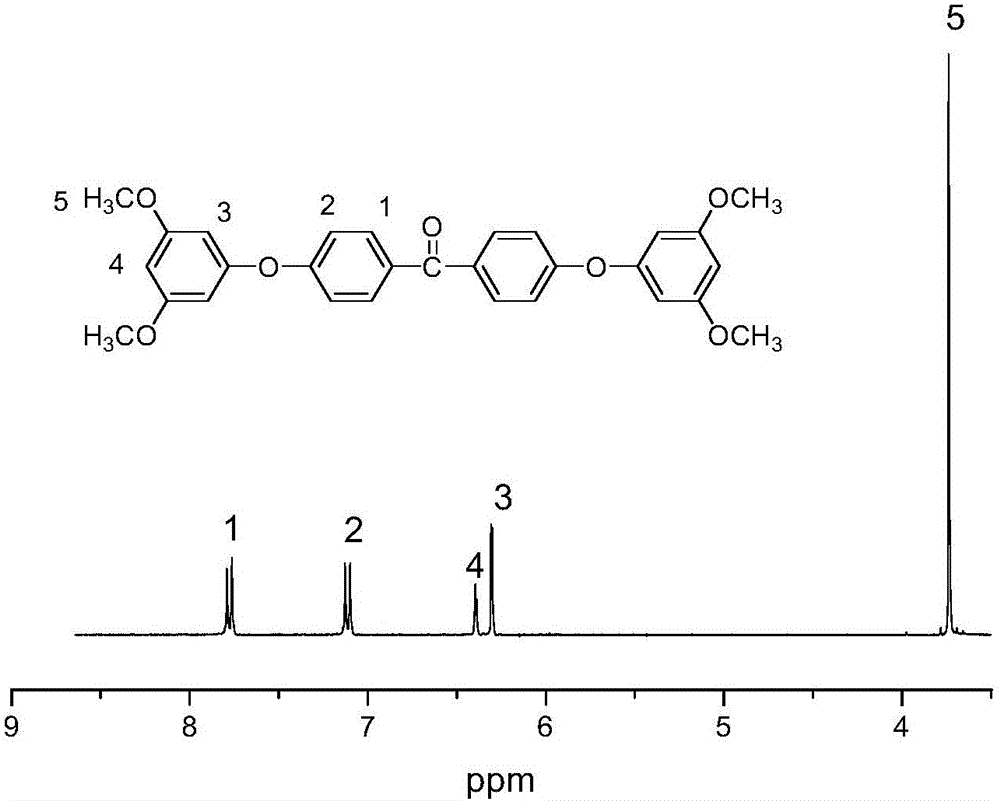
[0053] The first step: 0.10mol (15.4g) 3,5-dimethoxyphenol, 0.045mol (9.81g) 4,4'-difluorobenzophenone, 0.06mol (8.28g) potassium carbonate, 1.3mol ( Add 121mL) of N,N-dimethylacetamide and 0.4mol (42mL) of toluene into the reaction vessel equipped with a water-carrying device and a reflux condenser, heat to reflux with water for 2 hours under mechanical stirring and nitrogen protection, and then heat up Evaporate toluene at 150°C, continue to react at 130°C for 20 hours, cool down to room temperature, discharge the material in deionized water, wash with deionized water for 3 to 4 times until the filtrate is colorless, and vacuum dry at 80°C for 6 to 12 hours Recrystallize with a mixed solvent of water and acetone with a molar ratio of 1:4 to obtain light yellow 4,4'-bis(3,5-dimethoxyphenoxy)benzophenone;
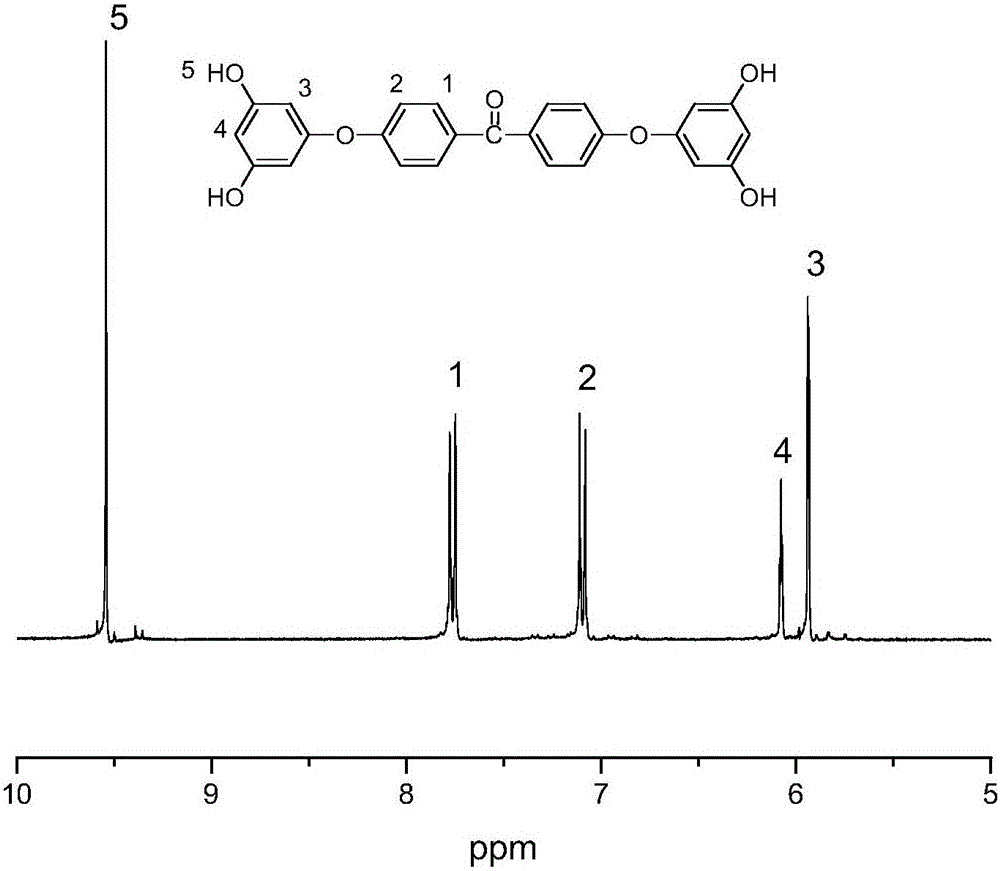
[0054] The second step: adding the 0.03mol (14.68g) 4,4'-bis[(3,5-dimethoxy)phenoxy]benzophenone into 0.6mol (38mL) dichloromethane to obtain a reaction solution , under m...
Embodiment 2
[0062]The difference between the first step of this embodiment and the first step of embodiment 1 is: the consumption of 4,4-difluorobenzophenone in embodiment 1 is changed to 0.035mol (10.05g), and the consumption of potassium carbonate is changed to 0.0525mol (7.25g), the amount of N,N-dimethylacetamide was changed to 1.12mol (104mL), the amount of toluene was changed to 0.42mol (44mL), the heating and reflux time was changed to 3 hours, and the reaction time was continued Change to 24 hours, and the mol ratio of recrystallization mixed solvent water and acetone is changed to 1:5;
[0063] The difference between the second step of this embodiment and the second step of Example 1 is that the consumption of dichloromethane is changed to 5.7mol (365mL), the consumption of boron tribromide is changed to 0.3mol (75.17g), and the consumption of methanol The dosage was changed to 0.9mol (36mL), the controlled reaction temperature of the liquid nitrogen-acetone system was changed to...
Embodiment 3
[0067] The difference between the first step of this example and the first step of Example 1 and Example 2 is that the amount of 4,4-difluorobenzophenone in Example 1 is changed to 0.050mol (10.9g), potassium carbonate The amount of N,N-dimethylacetamide was changed to 0.075mol (10.35g), the amount of N,N-dimethylacetamide was changed to 1.6mol (149mL), the amount of toluene was changed to 0.6mol (63mL), the time of reflux with water was changed to 3 hours, The reaction time at 150°C was changed to 18 hours, and the molar ratio of the recrystallization mixed solvent water and acetone was changed to 1:6;
[0068] The difference between the second step of this example and the second step of Example 1 and Example 2 is that the molar ratio of the recrystallization mixed solvent ethanol and water is changed to 1:0.1;
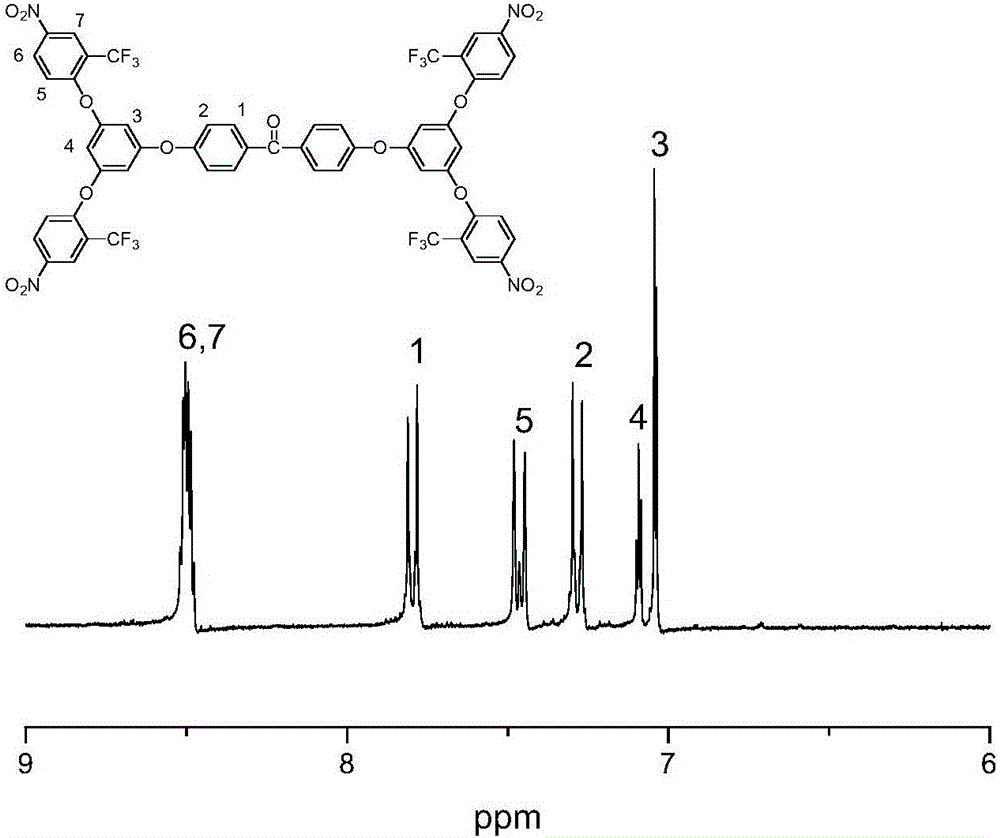
[0069] The difference between the third step of this embodiment and the third step of embodiment 1 and embodiment 2 is: the temperature for continuing the reaction i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com